|
Schlosser's Base
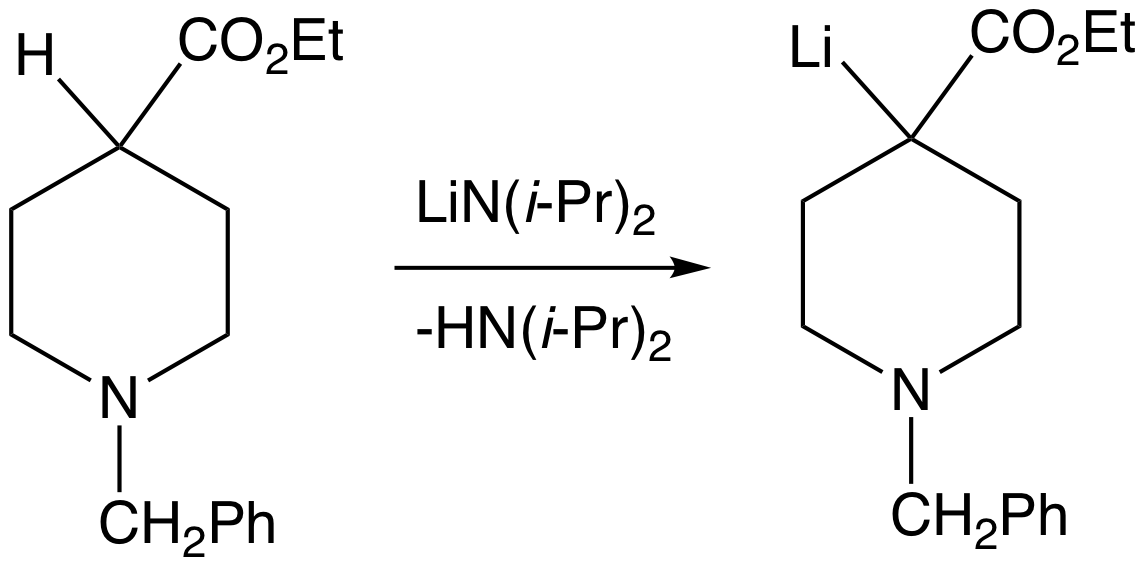
Schlosser's base (or Lochmann-Schlosser base) describes various superbasic mixtures of an alkyllithium compound and a potassium alkoxide. The reagent is named after Manfred Schlosser, although he uses the term ''LICKOR superbase'' (LIC denoting the alkyllithium, and KOR denoting the potassium alkoxide). The superbasic nature of the reagent is a consequence of the ''in situ'' formation of the corresponding organopotassium compound Organosodium chemistry is the chemistry of organometallic compounds containing a carbon to sodium chemical bond. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compounds, which are ..., as well as changes to the aggregation state of the alkyllithium species. Preparation and reactivity Commonly, the mixture called Schlosser's base is produced by combining ''n''-butyllithium and potassium ''tert''-butoxide in a one-to-one ratio. The high reactivity of Schlosser's base is exploited in s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superbase
A superbase is a compound that has a particularly high affinity for protons. Superbases are of theoretical interest and potentially valuable in organic synthesis. Superbases have been described and used since the 1850s.''Superbases for Organic Synthesis'' Ed. Ishikawa, T., John Wiley and Sons, Ltd.: West Sussex, UK. 2009. Definitions Generically IUPAC defines a superbase as a "compound having a very high basicity, such as lithium diisopropylamide." Superbases are often defined in two broad categories, organic and organometallic. Organic superbases are charge-neutral compounds with basicities greater than that of proton sponge (pKBH+ = 18.6 in MeCN)." In a related definition: any species with a higher absolute proton affinity (APA = 245.3 kcal/mol) and intrinsic gas phase basicity (GB = 239 kcal/mol) than proton sponge. Common superbases of this variety feature amidine, guanidine, and phosphazene functional groups. Strong superbases can be designed by utilizing multiple intram ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyllithium
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric. History and deve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands. Alkoxides, although generally not stable in protic solvents such as water, occur widely as intermediates in various reactions, including the Williamson ether synthesis. Transition metal alkoxides are widely used for coatings and as catalysts. Enolates are unsaturated alkoxides derived by deprotonation of a bond adjacent to a ketone or aldehyde. The nucleophilic center for simple alkoxides is located on the oxygen, whereas the nucleophilic site on enolates is delocalized onto both carbon and oxygen sites. Ynolates are also unsaturated alkoxides derived from acetylenic alcohols. Phenoxides are close relatives of the alkoxides, in which the alkyl group is replaced by a derivative of be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organopotassium Compound
Organosodium chemistry is the chemistry of organometallic compounds containing a carbon to sodium chemical bond. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compounds, which are commercially available and exhibit more convenient reactivity. The principal organosodium compound of commercial importance is sodium cyclopentadienide. Sodium tetraphenylborate can also be classified as an organosodium compound since in the solid state sodium is bound to the aryl groups. Organometal bonds in group 1 are characterised by high polarity with corresponding high nucleophilicity on carbon. This polarity results from the disparate electronegativity of carbon (2.55) and that of lithium 0.98, sodium 0.93 potassium 0.82 rubidium 0.82 caesium 0.79). The carbanionic nature of organosodium compounds can be minimized by resonance stabilization, for example, Ph3CNa. One consequence of the highly polarized Na-C bond is that simpl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-Butyllithium
''n''-Butyllithium C4H9Li (abbreviated ''n''-BuLi) is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene (SBS). Also, it is broadly employed as a strong base (superbase) in the synthesis of organic compounds as in the pharmaceutical industry. Butyllithium is commercially available as solutions (15%, 25%, 1.5 M, 2 M, 2.5 M, 10 M, etc.) in alkanes such as pentane, hexanes, and heptanes. Solutions in diethyl ether and THF can be prepared, but are not stable enough for storage. Annual worldwide production and consumption of butyllithium and other organolithium compounds is estimated at 2000 to 3000 tonnes. Although butyllithium is colorless, ''n''-butyllithium is usually encountered as a pale yellow solution in alkanes. Such solutions are stable indefinitely if properly stored,. but in practice, they degrade upon aging. Fine white precipitate (l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Tert-butoxide
Potassium ''tert''-butoxide is the chemical compound with the formula K+(CH3)3CO−. This colourless solid is a strong base (pKa of conjugate acid around 17), which is useful in organic synthesis. It exists as a tetrameric cubane-type cluster. It is often seen written in chemical literature as potassium ''t''-butoxide. The compound is often depicted as a salt, and it often behaves as such, but it is not ionized in solution. Preparation Potassium ''t''-butoxide is commercially available as a solution and as a solid, but it is often generated ''in situ'' for laboratory use because samples are so sensitive and older samples are often of poor quality. It is prepared by the reaction of dry ''tert''-butyl alcohol with potassium metal. The solid is obtained by evaporating these solutions followed by heating the solid. The solid can be purified by sublimation at 220 °C and 1 mmHg. Sublimation can also take place at 140 °C and 0.01 hPa. It is advisable to cover the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Benzyl
Benzylpotassium is an organopotassium compound with the formula C6H5CH2K. It is an orange powder. Like organo-alkali metal reagents in general, benzyl potassium is highly reactive, so much so that it reacts with most solvents. It is highly air sensitive. Synthesis One early synthesis proceeds by two-step transmetallation reaction via ''p''-tolylpotassium: :(CH3C6H4)2Hg + 2 K → 2 CH3C6H4K + Hg :CH3C6H4K → KCH2C6H5 A modern synthesis involves the reaction of butyllithium, potassium ''tert''-butoxide, and toluene.{{cite journal , doi = 10.1016/0022-328X(87)80117-1, title = Lithium-potassium exchange in alkyllithium/potassium t-pentoxide systems, journal = Journal of Organometallic Chemistry, volume = 326, pages = 1, year = 1987, last1 = Lochmann, first1 = L, last2 = Trekoval, first2 = J Although potassium hydride Potassium hydride, KH, is the inorganic compound of potassium and hydrogen. It is an alkali metal hydride. It is a white solid, although com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bases (chemistry)
Bases may refer to: *Bases (fashion), a military style of dress adopted by the chivalry of the sixteenth century *Business Association of Stanford Entrepreneurial Students (BASES) *the plural form of base (other) *the plural form of basis (other) Basis may refer to: Finance and accounting *Adjusted basis, the net cost of an asset after adjusting for various tax-related items *Basis point, 0.01%, often used in the context of interest rates * Basis trading, a trading strategy consisting o ... See also * Base (other) {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superbases
A superbase is a compound that has a particularly high affinity for protons. Superbases are of theoretical interest and potentially valuable in organic synthesis. Superbases have been described and used since the 1850s.''Superbases for Organic Synthesis'' Ed. Ishikawa, T., John Wiley and Sons, Ltd.: West Sussex, UK. 2009. Definitions Generically IUPAC defines a superbase as a "compound having a very high basicity, such as lithium diisopropylamide." Superbases are often defined in two broad categories, organic and organometallic. Organic superbases are charge-neutral compounds with basicities greater than that of proton sponge (pKBH+ = 18.6 in MeCN)." In a related definition: any species with a higher absolute proton affinity (APA = 245.3 kcal/mol) and intrinsic gas phase basicity (GB = 239 kcal/mol) than proton sponge. Common superbases of this variety feature amidine, guanidine, and phosphazene functional groups. Strong superbases can be designed by utilizing multiple intram ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |