|
Rule Of Mixtures
In materials science, a general rule of mixtures is a weighted mean used to predict various properties of a composite material . It provides a theoretical upper- and lower-bound on properties such as the elastic modulus, ultimate tensile strength, thermal conductivity, and electrical conductivity. In general there are two models, the ''rule of mixtures'' for axial loading (Voigt model), and the ''inverse rule of mixtures'' for transverse loading (Reuss model). For some material property E, the rule of mixtures states that the overall property in the direction parallel to the fibers could be as high as : E_\parallel = fE_f + \left(1-f\right)E_m The inverse rule of mixtures states that in the direction perpendicular to the fibers, the elastic modulus of a composite could be as low as :E_\perp = \left(\frac + \frac\right)^. where * f = \frac is the volume fraction of the fibers * E_\parallel is the material property of the composite parallel to the fibers * E_\perp is the materi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Composite Elastic Modulus
Composite or compositing may refer to: Materials * Composite material, a material that is made from several different substances ** Metal matrix composite, composed of metal and other parts ** Cermet, a composite of ceramic and metallic materials ** Dental composite, a substance used to fill cavities in teeth ** Composite armor, a type of tank armor * Alloy, a mixture of a metal and another element * Mixture, the combination of several different substances without chemical reaction Mathematics * Composite number, a positive integer that has at least one factor other than one or itself Science * Composite particle, a particle which is made up of smaller particles * ''Compositae'' or "composite family" of flowering plants * Composite volcano, a layered conical volcano * Compositing, another name for superposed epoch analysis, a statistical method used to analyze time series involving multiple events Technology * Compositing, combining of visual elements from separate sources ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Tension (physics)
Tension is the pulling or stretching force transmitted axially along an object such as a string, rope, chain, rod, truss member, or other object, so as to stretch or pull apart the object. In terms of force, it is the opposite of ''compression''. Tension might also be described as the action-reaction pair of forces acting at each end of an object. At the atomic level, when atoms or molecules are pulled apart from each other and gain potential energy with a restoring force still existing, the restoring force might create what is also called tension. Each end of a string or rod under such tension could pull on the object it is attached to, in order to restore the string/rod to its relaxed length. Tension (as a transmitted force, as an action-reaction pair of forces, or as a restoring force) is measured in newtons in the International System of Units (or pounds-force in Imperial units). The ends of a string or other object transmitting tension will exert forces on the objects ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Vegard's Law
In crystallography, materials science and metallurgy, Vegard's law is an empirical finding (heuristic approach) resembling the rule of mixtures. In 1921, Lars Vegard discovered that the lattice parameter of a solid solution of two constituents is approximately a weighted mean of the two constituents' lattice parameters at the same temperature: :a_ = (1-x)\ a_\mathrm + x\ a_\mathrm ''e.g.'', in the case of a mixed oxide of uranium and plutonium as used in the fabrication of MOX nuclear fuel: :a_\mathrm = 0.93\ a_\mathrm + 0.07\ a_\mathrm Vegard's law assumes that both components A and B in their pure form (''i.e.'', before mixing) have the same crystal structure. Here, is the lattice parameter of the solid solution, and are the lattice parameters of the pure constituents, and is the molar fraction of B in the solid solution. Vegard's law is seldom perfectly obeyed; often deviations from the linear behavior are observed. A detailed study of such deviations was conducted by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making up a substance. Thermometers are calibrated in various temperature scales that historically have relied on various reference points and thermometric substances for definition. The most common scales are the Celsius scale with the unit symbol °C (formerly called ''centigrade''), the Fahrenheit scale (°F), and the Kelvin scale (K), with the third being used predominantly for scientific purposes. The kelvin is one of the seven base units in the International System of Units (SI). Absolute zero, i.e., zero kelvin or −273.15 °C, is the lowest point in the thermodynamic temperature scale. Experimentally, it can be approached very closely but not actually reached, as recognized in the third law of thermodynamics. It would be impossible ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Richmann's Law
Richmann's law, sometimes referred to as Richmann's rule, Richmann's mixing rule, Richmann's rule of mixture or Richmann's law of mixture, is a physical law for calculating the mixing temperature when pooling multiple bodies. It is named after the Baltic German physicist Georg Wilhelm Richmann, who published the relationship in 1750, establishing the first general equation for calorimetric calculations. Origin Through experimental measurements, Wilhelm Richmann determined that the following relationship holds when water of different temperatures is mixed: : m_ \cdot T_1 + m_2 \cdot T_2 = (m_ + m_)\cdot T_\mathrm It follows: Here m_1and m_2 are the masses of the two mixture components, T_1 and T_2 are their respective initial temperatures, and T_m is the mixture temperature. This observation is called ''Richmann's law'' in the narrower sense and applies in principle to all substances of the same state of aggregation. According to this, the mixing temperature is the weighted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Mass Fraction (chemistry)
In chemistry, the mass fraction of a substance within a mixture is the ratio w_i (alternatively denoted Y_i) of the mass m_i of that substance to the total mass m_\text of the mixture. Expressed as a formula, the mass fraction is: : w_i = \frac . Because the individual masses of the ingredients of a mixture sum to m_\text, their mass fractions sum to unity: : \sum_^ w_i = 1. Mass fraction can also be expressed, with a denominator of 100, as percentage by mass (in commercial contexts often called ''percentage by weight'', abbreviated ''wt.%'' or ''% w/w''; see mass versus weight). It is one way of expressing the composition of a mixture in a dimensionless size; mole fraction (percentage by moles, mol%) and volume fraction ( percentage by volume, vol%) are others. When the prevalences of interest are those of individual chemical elements, rather than of compounds or other substances, the term ''mass fraction'' can also refer to the ratio of the mass of an element to the tot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Kopp's Law
Kopp's law can refer to either of two relationships discovered by the German chemist Hermann Franz Moritz Kopp (1817–1892). #Kopp found "that the molecular heat capacity of a solid compound is the sum of the atomic heat capacities of the elements composing it; the elements having atomic heat capacities lower than those required by the Dulong–Petit law retain these lower values in their compounds." #In studying organic compounds, Kopp found a regular relationship between boiling points and the number of CH2 groups present.See page 942 of Kopp–Neumann law The Kopp–Neumann law, named for Kopp and Franz Ernst Neumann, is a common approach for determining the specific heat ''C'' (in J·kg−1·K−1) of compounds using the following equation: C = \sum_^N C_i f_i, where ''N'' is the total number of compound constituents, and ''Ci'' and ''fi'' denote the specific heat and Mass fraction (chemistry), mass fraction of the ''i''-th constituent. This law works surprisingly well a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Gladstone–Dale Relation
The Gladstone–Dale relation is a mathematical relation used for optical analysis of liquids, the determination of composition from optical measurements. It can also be used to calculate the density of a liquid for use in fluid dynamics (e.g., flow visualization). The relation has also been used to calculate refractive index of glass and minerals in optical mineralogy. Uses In the Gladstone–Dale relation, (n-1)/\rho = \sum km, the index of refraction (''n'') or the density (''ρ'' in g/cm3) of miscible liquids that are mixed in mass fraction (''m'') can be calculated from characteristic optical constants (the molar refractivity ''k'' in cm3/g) of pure molecular end-members. For example, for any mass (''m'') of ethanol added to a mass of water, the alcohol content is determined by measuring density or index of refraction (Brix refractometer). Mass (''m'') per unit volume (''V'') is the density ''m''/''V''. Mass is conserved on mixing, but the volume of 1 cm3 of ethanol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Amagat's Law
Amagat's law or the law of partial volumes describes the behaviour and properties of mixtures of ideal (as well as some cases of non-ideal) gases. It is of use in chemistry and thermodynamics. It is named after Émile Amagat. Overview Amagat's law states that the extensive volume of a gas mixture is equal to the sum of volumes of the component gases, if the temperature and the pressure remain the same: N\, v(T, p) = \sum_^K N_i\, v_i(T, p). This is the experimental expression of volume as an extensive quantity. According to Amagat's law of partial volume, the total volume of a non-reacting mixture of gases at constant temperature and pressure should be equal to the sum of the individual partial volumes of the constituent gases. So if V_1, V_2, \dots, V_n are considered to be the partial volumes of components in the gaseous mixture, then the total volume would be represented as :V = V_1 + V_2 + V_3 + \dots + V_n = \sum_i V_i. Both Amagat's and Dalton's law predict the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Mass Density
Density (volumetric mass density or specific mass) is the ratio of a substance's mass to its volume. The symbol most often used for density is ''ρ'' (the lower case Greek language, Greek letter rho), although the Latin letter ''D'' (or ''d'') can also be used: \rho = \frac, where ''ρ'' is the density, ''m'' is the mass, and ''V'' is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume, although this is scientifically inaccurate this quantity is more specifically called specific weight. For a pure substance, the density is equal to its mass concentration (chemistry), mass concentration. Different materials usually have different densities, and density may be relevant to buoyancy, purity and packaging. Osmium is the densest known element at standard conditions for temperature and pressure. To simplify comparisons of density across different systems of units, it is sometimes replaced by t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Poisson's Ratio
In materials science and solid mechanics, Poisson's ratio (symbol: ( nu)) is a measure of the Poisson effect, the deformation (expansion or contraction) of a material in directions perpendicular to the specific direction of loading. The value of Poisson's ratio is the negative of the ratio of transverse strain to axial strain. For small values of these changes, is the amount of transversal elongation divided by the amount of axial compression. Most materials have Poisson's ratio values ranging between 0.0 and 0.5. For soft materials, such as rubber, where the bulk modulus is much higher than the shear modulus, Poisson's ratio is near 0.5. For open-cell polymer foams, Poisson's ratio is near zero, since the cells tend to collapse in compression. Many typical solids have Poisson's ratios in the range of 0.2 to 0.3. The ratio is named after the French mathematician and physicist Siméon Poisson. Origin Poisson's ratio is a measure of the Poisson effect, the phenomenon in whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
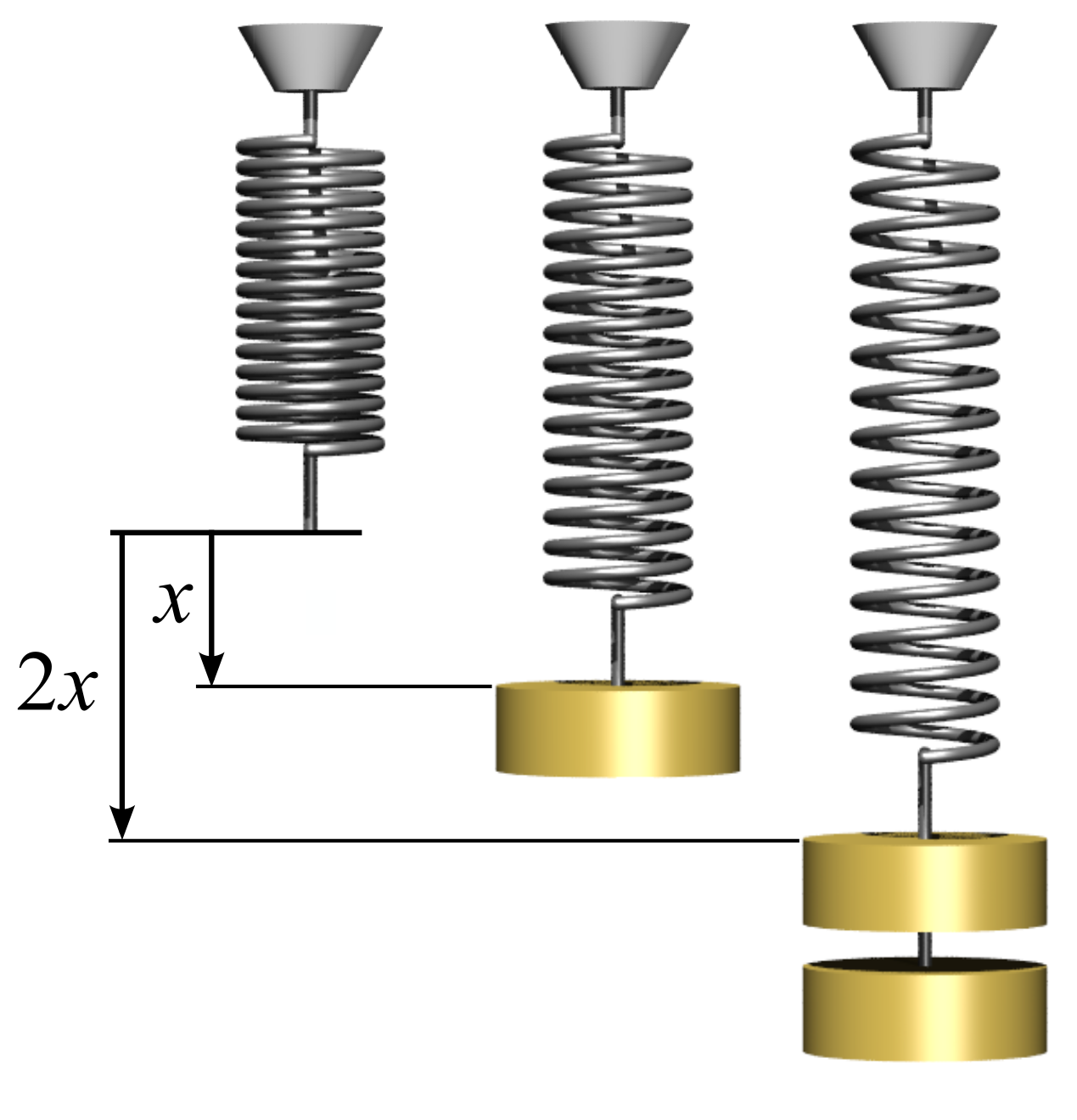
Hooke's Law
In physics, Hooke's law is an empirical law which states that the force () needed to extend or compress a spring by some distance () scales linearly with respect to that distance—that is, where is a constant factor characteristic of the spring (i.e., its stiffness), and is small compared to the total possible deformation of the spring. The law is named after 17th-century British physicist Robert Hooke. He first stated the law in 1676 as a Latin anagram. He published the solution of his anagram in 1678 as: ("as the extension, so the force" or "the extension is proportional to the force"). Hooke states in the 1678 work that he was aware of the law since 1660. Hooke's equation holds (to some extent) in many other situations where an elastic body is deformed, such as wind blowing on a tall building, and a musician plucking a string of a guitar. An elastic body or material for which this equation can be assumed is said to be linear-elastic or Hookean. Hooke's law is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |