|
Rudolf K. Allemann
Professor Rudolf Konrad Allemann is a Distinguished Research Professor and Pro Vice-Chancellor International and Student Recruitment and Head of the College of Physical Sciences and Engineering at Cardiff University. Allemann joined Cardiff University in 2005, after working at the University of Birmingham, the Swiss Federal Institute of Technology ETH Zurich and the UK MRC National Institute for Medical Research at Mill Hill. He was previously Head of the School of Chemistry at Cardiff University until April 2017. Education and academic career Allemann earned his Dipl. Chem. ETH (B.S./M.S.) from ETH Zurich in 1985. His PhD was carried out at Harvard University and ETH Zurich with Steven A. Benner and culminated in the award of a Dr. sc. nat ETH for his thesis 'Evolutionary Guidance as a Tool in Organic Chemistry'. He then moved to the UK to as a Royal Society and Swiss National Science Foundation postdoctoral fellow at the National Institute for Medical Research, before returni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Switzerland
). Swiss law does not designate a ''capital'' as such, but the federal parliament and government are installed in Bern, while other federal institutions, such as the federal courts, are in other cities (Bellinzona, Lausanne, Luzern, Neuchâtel, St. Gallen a.o.). , coordinates = , largest_city = Zürich , official_languages = , englishmotto = "One for all, all for one" , religion_year = 2020 , religion_ref = , religion = , demonym = , german: Schweizer/Schweizerin, french: Suisse/Suissesse, it, svizzero/svizzera or , rm, Svizzer/Svizra , government_type = Federalism, Federal assembly-independent Directorial system, directorial republic with elements of a direct democracy , leader_title1 = Federal Council (Switzerland), Federal Council , leader_name1 = , leader_title2 = , leader_name2 = Walter Thurnherr , legislature = Fe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (included in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terpene
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n > 1. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes are further classified by the number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), as examples. The terpene alpha-pinene, is a major component of the common solvent, turpentine. History and terminology The term ''terpene'' was coined in 1866 by the German chemist August Kekulé to denote all hydrocarbons having the empirical formula C10H16, of which camphene was one. Previously, many hydrocarbons having the empirical formula C10H16 had been called "camphene", but many other hydrocarbons of the same composition had had different names. Kekulé coined the term "terpene" in order to reduce the confusion. The name "terpene" is a shortened form of "terpentine", an obsolete spelling of "turpentine". Although sometimes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydrofolate Reductase
Dihydrofolate reductase, or DHFR, is an enzyme that reduces dihydrofolic acid to tetrahydrofolic acid, using NADPH as an electron donor, which can be converted to the kinds of tetrahydrofolate cofactors used in 1-carbon transfer chemistry. In humans, the DHFR enzyme is encoded by the ''DHFR'' gene. It is found in the q11→q22 region of chromosome 5. Bacterial species possess distinct DHFR enzymes (based on their pattern of binding diaminoheterocyclic molecules), but mammalian DHFRs are highly similar. Structure A central eight-stranded beta-pleated sheet makes up the main feature of the polypeptide backbone folding of DHFR. Seven of these strands are parallel and the eighth runs antiparallel. Four alpha helices connect successive beta strands. Residues 9 – 24 are termed "Met20" or "loop 1" and, along with other loops, are part of the major subdomain that surround the active site. The active site is situated in the N-terminal half of the sequence, which includes a conse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoactivated Peptide
Photoactivated peptides are modified natural or synthetic peptides the functions of which can be activated with light. This can be done either irreversibly or in a reversible way. Caged peptides which contain photocleavable protecting groups belong to irreversibly activated peptides. Reversible activation/deactivation of peptide function are achieved by incorporation photo-controllable fragments (molecular photoswitches) in the side chains or in the backbone of peptide templates to get the photo-controlled peptides, which can reversibly change their structure upon irradiation with light of different wavelength. As the consequence, the properties, function and biological activity of the modified peptides can be controlled by light. Since light can be directed to specific areas, such peptides can be activated only at targeted sites. Azobenzenes, and diarylethenes can be used as the photoswitches. For therapeutic use, photoswitches with longer wavelengths (near-infrared, to penetrate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Biology
Synthetic biology (SynBio) is a multidisciplinary area of research that seeks to create new biological parts, devices, and systems, or to redesign systems that are already found in nature. It is a branch of science that encompasses a broad range of methodologies from various disciplines, such as biotechnology, biomaterials, material science/engineering, genetic engineering, molecular biology, molecular engineering, systems biology, membrane science, biophysics, chemical and biological engineering, electrical and computer engineering, control engineering and evolutionary biology. Due to more powerful genetic engineering capabilities and decreased DNA synthesis and sequencing costs, the field of synthetic biology is rapidly growing. In 2016, more than 350 companies across 40 countries were actively engaged in synthetic biology applications; all these companies had an estimated net worth of $3.9 billion in the global market. Definition Synthetic biology currently has no gen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Dynamics
Proteins are generally thought to adopt unique structures determined by their amino acid sequences. However, proteins are not strictly static objects, but rather populate ensembles of (sometimes similar) conformations. Transitions between these states occur on a variety of length scales (tenths of Å to nm) and time scales (ns to s), and have been linked to functionally relevant phenomena such as allosteric signaling and enzyme catalysis. The study of protein dynamics is most directly concerned with the transitions between these states, but can also involve the nature and equilibrium populations of the states themselves. These two perspectives—kinetics and thermodynamics, respectively—can be conceptually synthesized in an "energy landscape" paradigm: highly populated states and the kinetics of transitions between them can be described by the depths of energy wells and the heights of energy barriers, respectively. Local flexibility: atoms and residues Portions of protein s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Mechanical Tunnelling
Quantum tunnelling, also known as tunneling (American English, US) is a quantum mechanics, quantum mechanical phenomenon whereby a wavefunction can propagate through a potential barrier. The transmission through the barrier can be finite and depends exponentially on the barrier height and barrier width. The wavefunction may disappear on one side and reappear on the other side. The wavefunction and its first derivative are Continuous function, continuous. In steady-state, the probability flux in the forward direction is spatially uniform. No particle or wave is lost. Tunneling occurs with barriers of thickness around 1–3 nm and smaller. Some authors also identify the mere penetration of the wavefunction into the barrier, without transmission on the other side as a tunneling effect. Quantum tunneling is not predicted by the laws of classical mechanics where surmounting a potential barrier requires sufficient kinetic energy. Quantum tunneling plays an essential role in phys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydrofolate Reductase
Dihydrofolate reductase, or DHFR, is an enzyme that reduces dihydrofolic acid to tetrahydrofolic acid, using NADPH as an electron donor, which can be converted to the kinds of tetrahydrofolate cofactors used in 1-carbon transfer chemistry. In humans, the DHFR enzyme is encoded by the ''DHFR'' gene. It is found in the q11→q22 region of chromosome 5. Bacterial species possess distinct DHFR enzymes (based on their pattern of binding diaminoheterocyclic molecules), but mammalian DHFRs are highly similar. Structure A central eight-stranded beta-pleated sheet makes up the main feature of the polypeptide backbone folding of DHFR. Seven of these strands are parallel and the eighth runs antiparallel. Four alpha helices connect successive beta strands. Residues 9 – 24 are termed "Met20" or "loop 1" and, along with other loops, are part of the major subdomain that surround the active site. The active site is situated in the N-terminal half of the sequence, which includes a conse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terpenoid
The terpenoids, also known as isoprenoids, are a class of naturally occurring organic chemicals derived from the 5-carbon compound isoprene and its derivatives called terpenes, diterpenes, etc. While sometimes used interchangeably with "terpenes", terpenoids contain additional functional groups, usually containing oxygen. When combined with the hydrocarbon terpenes, terpenoids comprise about 80,000 compounds. They are the largest class of plant secondary metabolites, representing about 60% of known natural products. Many terpenoids have substantial pharmacological bioactivity and are therefore of interest to medicinal chemists. Plant terpenoids are used for their aromatic qualities and play a role in traditional herbal remedies. Terpenoids contribute to the scent of eucalyptus, the flavors of cinnamon, cloves, and ginger, the yellow color in sunflowers, and the red color in tomatoes. Well-known terpenoids include citral, menthol, camphor, salvinorin A in the plant '' Salvia di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terpene
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n > 1. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes are further classified by the number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), as examples. The terpene alpha-pinene, is a major component of the common solvent, turpentine. History and terminology The term ''terpene'' was coined in 1866 by the German chemist August Kekulé to denote all hydrocarbons having the empirical formula C10H16, of which camphene was one. Previously, many hydrocarbons having the empirical formula C10H16 had been called "camphene", but many other hydrocarbons of the same composition had had different names. Kekulé coined the term "terpene" in order to reduce the confusion. The name "terpene" is a shortened form of "terpentine", an obsolete spelling of "turpentine". Although sometimes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
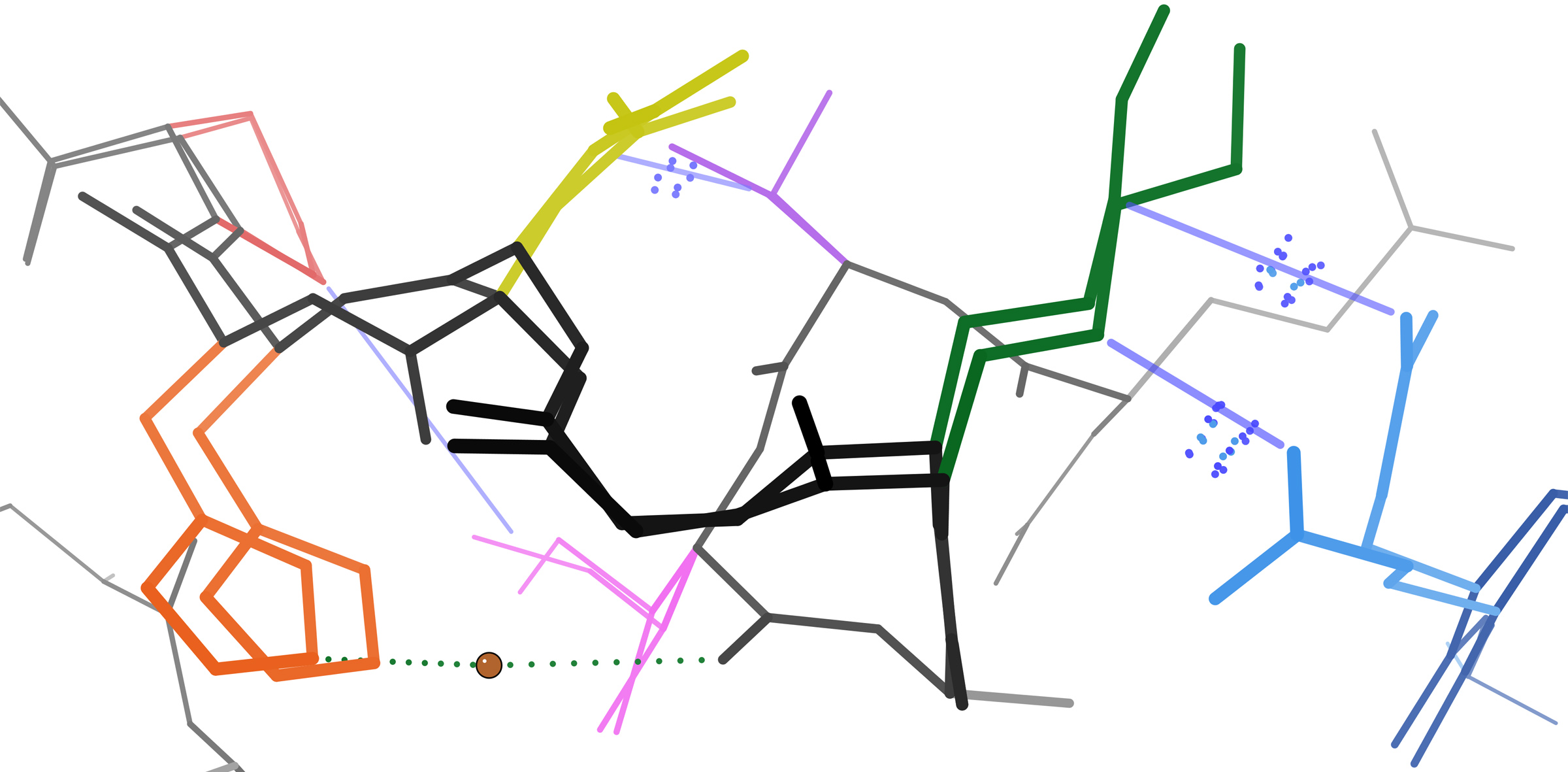
Delta-cadinene Synthase
The enzyme (+)-δ-cadinene synthase (EC 4.2.3.13) catalyzes the chemical reaction :(2''E'',6''E'')-farnesyl diphosphate \rightleftharpoons (+)-δ-cadinene + diphosphate This enzyme belongs to the family of lyases, specifically those carbon-oxygen lyases acting on phosphates. The systematic name of this enzyme class is (2''E'',6''E'')-farnesyl-diphosphate diphosphate-lyase (cyclizing, (+)-δ-cadinene-forming). This enzyme participates in terpenoid biosynthesis. It employs one cofactor, magnesium. δ-Cadinene synthase, a sesquiterpene cyclase, is an enzyme expressed in plants that catalyzes a cyclization reaction in terpenoid biosynthesis. The enzyme cyclizes farnesyl diphosphate to δ-cadinene and releases pyrophosphate. δ-Cadinene synthase is one of the key steps in the synthesis of gossypol, a toxic terpenoid produced in cotton seeds. Recently, cotton plants that stably underexpress the enzyme in seeds have been developed using RNA interference techniques, producing a plant ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |





.png)