|
Ribophorin
''Ribophorins'' are dome shaped transmembrane glycoproteins which are located in the membrane of the rough endoplasmic reticulum, but are absent in the membrane of the smooth endoplasmic reticulum. There are two types of ribophorines: ribophorin I and II. These act in the protein complex oligosaccharyltransferase (OST) as two different subunits of the named complex. Ribophorin I and II are only present in eukaryote cells. Both types of ribophorins develop a key role in the binding of ribosomes to the rough endoplasmic reticulum as well as in the co-translational processes that depend on this interaction. The content of ribophorin of the rough endoplasmic reticulum is equal to the stoichiometric number of ribosomal units. Therefore, this suggests the great importance, abundance and good preservation of these proteins in the reticulum. Consequently, defects in the genes that encode these proteins may cause congenital disorders and devastating consequences; ribophorin I and II are en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ribophorin Is A Subunit Of Oligosaccharide Transferasa In The RER
''Ribophorins'' are dome shaped transmembrane glycoproteins which are located in the membrane of the rough endoplasmic reticulum, but are absent in the membrane of the smooth endoplasmic reticulum. There are two types of ribophorines: ribophorin I and II. These act in the protein complex oligosaccharyltransferase (OST) as two different subunits of the named complex. Ribophorin I and II are only present in eukaryote cells. Both types of ribophorins develop a key role in the binding of ribosomes to the rough endoplasmic reticulum as well as in the co-translational processes that depend on this interaction. The content of ribophorin of the rough endoplasmic reticulum is equal to the stoichiometric number of ribosomal units. Therefore, this suggests the great importance, abundance and good preservation of these proteins in the reticulum. Consequently, defects in the genes that encode these proteins may cause congenital disorders and devastating consequences; ribophorin I and II are en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycoproteins
Glycoproteins are proteins which contain oligosaccharide chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycosylation. Secreted extracellular proteins are often glycosylated. In proteins that have segments extending extracellularly, the extracellular segments are also often glycosylated. Glycoproteins are also often important integral membrane proteins, where they play a role in cell–cell interactions. It is important to distinguish endoplasmic reticulum-based glycosylation of the secretory system from reversible cytosolic-nuclear glycosylation. Glycoproteins of the cytosol and nucleus can be modified through the reversible addition of a single GlcNAc residue that is considered reciprocal to phosphorylation and the functions of these are likely to be an additional regulatory mechanism that controls phosphorylation-based signalling. In contrast, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RPN2
Dolichyl-diphosphooligosaccharide—protein glycosyltransferase subunit 2, also called ribophorin ǁ is an enzyme that in humans is encoded by the ''RPN2'' gene. Function This gene encodes a type I integral ribophorin membrane protein found only in the rough endoplasmic reticulum. The encoded protein is part of an N-oligosaccharyl transferase complex that links high mannose oligosaccharides to asparagine residues found in the Asn-X-Ser/Thr consensus motif of nascent polypeptide chains. This protein is similar in sequence to the yeast oligosaccharyl transferase subunit SWP1. RPN2 has been demonstrated to be a prognostic marker of human cancer, and may be a potential target of clinical importance. Structure Gene The ''RPN2'' gene lies on the chromosome location of 20q11.23 and consists of 19 exons. Protein RPN2 consists of 631 amino acid residues and weighs 69284Da. Function RPN2 is a unique integral glycoprotein in rough ER membrane that is involved in translocation an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycoproteins
Glycoproteins are proteins which contain oligosaccharide chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycosylation. Secreted extracellular proteins are often glycosylated. In proteins that have segments extending extracellularly, the extracellular segments are also often glycosylated. Glycoproteins are also often important integral membrane proteins, where they play a role in cell–cell interactions. It is important to distinguish endoplasmic reticulum-based glycosylation of the secretory system from reversible cytosolic-nuclear glycosylation. Glycoproteins of the cytosol and nucleus can be modified through the reversible addition of a single GlcNAc residue that is considered reciprocal to phosphorylation and the functions of these are likely to be an additional regulatory mechanism that controls phosphorylation-based signalling. In contrast, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
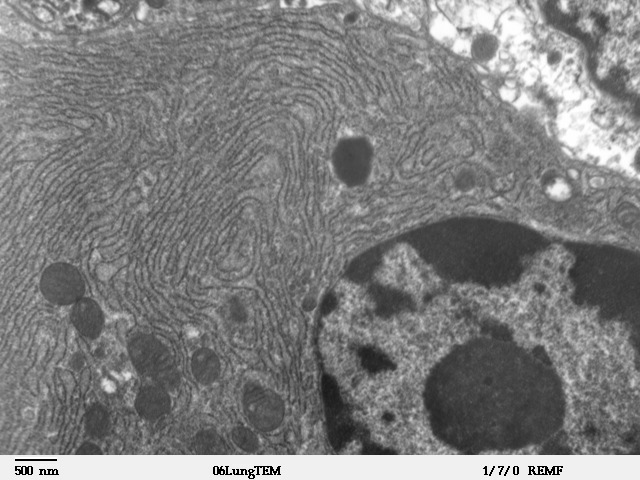
Smooth Endoplasmic Reticulum
The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae (in the RER), and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa. The two types of ER share many of the same proteins and engage in certain common activities such as the synthesis of certain lipids and cholesterol. Different types of cells contain different ratios of the two types of ER depending on the activities of the cell. RER is found mainly toward the nucleus of cell and SER towards the cell membrane or plasma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ribosomes
Ribosomes ( ) are macromolecular machines, found within all cells, that perform biological protein synthesis (mRNA translation). Ribosomes link amino acids together in the order specified by the codons of messenger RNA (mRNA) molecules to form polypeptide chains. Ribosomes consist of two major components: the small and large ribosomal subunits. Each subunit consists of one or more ribosomal RNA (rRNA) molecules and many ribosomal proteins (RPs or r-proteins). The ribosomes and associated molecules are also known as the ''translational apparatus''. Overview The sequence of DNA that encodes the sequence of the amino acids in a protein is transcribed into a messenger RNA chain. Ribosomes bind to messenger RNAs and use their sequences for determining the correct sequence of amino acids to generate a given protein. Amino acids are selected and carried to the ribosome by transfer RNA (tRNA) molecules, which enter the ribosome and bind to the messenger RNA chain via an anti-cod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RPN1
Dolichyl-diphosphooligosaccharide—protein glycosyltransferase subunit 1 is an enzyme that in humans is encoded by the ''RPN1'' gene. This gene encodes a type I integral ribophorin ''Ribophorins'' are dome shaped transmembrane glycoproteins which are located in the membrane of the rough endoplasmic reticulum, but are absent in the membrane of the smooth endoplasmic reticulum. There are two types of ribophorines: ribophorin I a ... membrane protein found only in the rough endoplasmic reticulum. The encoded protein is part of an N-oligosaccharyl transferase complex that links high mannose oligosaccharides to asparagine residues found in the Asn-X-Ser/Thr consensus motif of nascent polypeptide chains. This protein should not be confused with RPN1 of yeast, Drosophila, and C. elegans, which forms part of the regulatory subunit of the 26S proteasome and may mediate binding of ubiquitin-like domains to this proteasome. The human version of this proteasome subunit is PSMD2. References ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aminoacids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytoplasm
In cell biology, the cytoplasm is all of the material within a eukaryotic cell, enclosed by the cell membrane, except for the cell nucleus. The material inside the nucleus and contained within the nuclear membrane is termed the nucleoplasm. The main components of the cytoplasm are cytosol (a gel-like substance), the organelles (the cell's internal sub-structures), and various cytoplasmic inclusions. The cytoplasm is about 80% water and is usually colorless. The submicroscopic ground cell substance or cytoplasmic matrix which remains after exclusion of the cell organelles and particles is groundplasm. It is the hyaloplasm of light microscopy, a highly complex, polyphasic system in which all resolvable cytoplasmic elements are suspended, including the larger organelles such as the ribosomes, mitochondria, the plant plastids, lipid droplets, and vacuoles. Most cellular activities take place within the cytoplasm, such as many metabolic pathways including glycolysis, and proces ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Asparagine
Asparagine (symbol Asn or N) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain carboxamide, classifying it as a polar (at physiological pH), aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it. It is encoded by the codons AAU and AAC. History Asparagine was first isolated in 1806 in a crystalline form by French chemists Louis Nicolas Vauquelin and Pierre Jean Robiquet (then a young assistant). It was isolated from asparagus juice, in which it is abundant, hence the chosen name. It was the first amino acid to be isolated. Three years later, in 1809, Pierre Jean Robiquet identified a substance from liquorice root with properties which he qualified as very similar to those of asparagine, and which Plisson identi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-glycosylation
''N''-linked glycosylation, is the attachment of an oligosaccharide, a carbohydrate consisting of several sugar molecules, sometimes also referred to as glycan, to a nitrogen atom (the amide nitrogen of an asparagine (Asn) residue of a protein), in a process called ''N''-glycosylation, studied in biochemistry. This type of linkage is important for both the structure and function of many eukaryotic proteins. The ''N''-linked glycosylation process occurs in eukaryotes and widely in archaea, but very rarely in bacteria. The nature of ''N''-linked glycans attached to a glycoprotein is determined by the protein and the cell in which it is expressed. It also varies across species. Different species synthesize different types of ''N''-linked glycan. Energetics of bond formation There are two types of bonds involved in a glycoprotein: bonds between the saccharides residues in the glycan and the linkage between the glycan chain and the protein molecule. The sugar moieties are linked t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |