|
Rhamnose
Rhamnose (Rha, Rham) is a naturally occurring deoxy sugar. It can be classified as either a methyl-pentose or a 6-deoxy-hexose. Rhamnose predominantly occurs in nature in its L-form as L-rhamnose (6-deoxy-L-mannose). This is unusual, since most of the naturally occurring sugars are in D-form. Exceptions are the methyl pentoses L-fucose and L-rhamnose and the pentose L-arabinose. However, examples of naturally-occurring D-rhamnose include some species of bacteria, such as ''Pseudomonas aeruginosa'' and ''Helicobacter pylori''. Rhamnose can be isolated from Buckthorn (''Rhamnus''), poison sumac, and plants in the genus ''Uncaria''. Rhamnose is also produced by microalgae belonging to class Bacillariophyceae (diatoms). Rhamnose is commonly bound to other sugars in nature. It is a common glycone component of glycosides from many plants. Rhamnose is also a component of the outer cell membrane of acid-fast bacteria in the ''Mycobacterium'' genus, which includes the organism that cau ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galactose Binding Lectin Domain
In molecular biology, the galactose binding lectin domain is a protein domain. It is found in many proteins including the lectin purified from sea urchin (''Anthocidaris crassispina'') eggs, SUEL. This lectin exists as a disulfide-linked homodimer of two subunits; the dimeric form is essential for hemagglutination activity. The sea urchin egg lectin (SUEL) forms a new class of lectins. Although SUEL was first isolated as a D-galactoside binding lectin, it was later shown that it binds to L-rhamnose preferentially. L-rhamnose and D-galactose share the same hydroxyl group orientation at C2 and C4 of the pyranose ring structure. A cysteine-rich domain (the galactose binding lectin domain) homologous to the SUEL protein has been identified in the following proteins: *Plant beta-galactosidases (lactases). *Mammalian latrophilin, the calcium independent receptor of alpha-latrotoxin (CIRL). The galactose-binding lectin domain is not required for alpha-latratoxin binding. **Human lat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-L-rhamnosidase
Alpha-L-rhamnosidase (, ''alpha-L-rhamnosidase T'', ''alpha-L-rhamnosidase N'') is an enzyme with systematic name ''alpha-L-rhamnoside rhamnohydrolase''. This enzyme catalyses the following chemical reaction : Hydrolysis Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ... of terminal non-reducing alpha-L- rhamnose residues in alpha-L-rhamnosides References External links * {{Portal bar, Biology, border=no EC 3.2.1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycoside
In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of ''Heliconius'' butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body. In formal terms, a glycoside is any molecule in which a sugar group is bonded through its anomeric carbon to another group via a glycosidic bond. Glycosides can be linked by an O- (an ''O-glycoside''), N- (a ''glycosylamine''), S-(a ''thioglycoside''), or C- (a '' C-glycoside'') glycosidic bond. According to th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Robinose
Robinose is a disaccharide composed of 6″-O-α- rhamnopyranosyl-β- galactopyranoside. The sugar can be found in ''Acalypha hispida''. Robinin is a kaempferol Kaempferol (3,4′,5,7-tetrahydroxyflavone) is a natural flavonol, a type of flavonoid, found in a variety of plants and plant-derived foods including kale, beans, tea, spinach, and broccoli. Kaempferol is a yellow crystalline solid with a meltin ...-3-''O''-robinoside-7-''O''-rhamnoside. References Disaccharides Deoxy sugars {{biochemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexose
In chemistry, a hexose is a monosaccharide (simple sugar) with six carbon atoms. The chemical formula for all hexoses is C6H12O6, and their molecular weight is 180.156 g/mol. Hexoses exist in two forms, open-chain or cyclic, that easily convert into each other in aqueous solutions. The open-chain form of a hexose, which usually is favored in solutions, has the general structure H–(CHOH)''n''−1–C(=O)–(CHOH)4−''n''–H, where ''n'' is 1, 2, or 3. Namely, five of the carbons have one hydroxyl functional group (–OH) each, connected by a single bond, and one has an oxo group (=O), forming a carbonyl group (C=O). The remaining bonds of the carbon atoms are satisfied by seven hydrogen atoms. The carbons are commonly numbered 1 to 6 starting at the end closest to the carbonyl. Hexoses are extremely important in biochemistry, both as isolated molecules (such as glucose and fructose) and as building blocks of other compounds such as starch, cellulose, and glycosides. Hexose ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rutinose
Rutinose is the disaccharide also known as 6-''O''-α-L-rhamnose, rhamnosyl-D-glucose (C12H22O10) that is present in some flavonoid glycosides. It is prepared from rutin by hydrolysis with the enzyme rhamnodiastase. References * Disaccharides Deoxy sugars {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tuberculosis
Tuberculosis (TB) is an infectious disease usually caused by '' Mycobacterium tuberculosis'' (MTB) bacteria. Tuberculosis generally affects the lungs, but it can also affect other parts of the body. Most infections show no symptoms, in which case it is known as latent tuberculosis. Around 10% of latent infections progress to active disease which, if left untreated, kill about half of those affected. Typical symptoms of active TB are chronic cough with blood-containing mucus, fever, night sweats, and weight loss. It was historically referred to as consumption due to the weight loss associated with the disease. Infection of other organs can cause a wide range of symptoms. Tuberculosis is spread from one person to the next through the air when people who have active TB in their lungs cough, spit, speak, or sneeze. People with Latent TB do not spread the disease. Active infection occurs more often in people with HIV/AIDS and in those who smoke. Diagnosis of active TB is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neohesperidose
Neohesperidose is the disaccharide which is present in some flavonoids. It can be found in species of ''Typha.'' ''Delphinidin-3-neohesperidoside and cyanidin-3- neohesperidoside from receptacles of Podocarpus species, Oyvind M. Andersen, Phytochemistry, 1989, Volume 28, Issue 2, Pages 495–497, Neohesperidosides * Cyanidin-3-neohesperidoside * Delphinidin-3-neohesperidoside * Rhoifolin or apigenin 7-''O''-neohesperidoside * Myricetin-3-''O''-neohesperidoside found in ''Physalis angulata''A novel cytotoxic flavonoid glycoside from Physalis angulata. N. Ismail and M. Alam, Fitoterapia, Volume 72, Issue 6, August 2001, Pages 676-679, * Neohesperidin (hesperetin 7-''O''-neohesperidoside) * Neoeriocitrin (eriodictyol Eriodictyol is a bitter-masking flavanone, a flavonoid extracted from yerba santa (''Eriodictyon californicum''), a plant native to North America. Eriodictyol is one of the four flavanones identified in this plant as having taste-modifying proper ... 7-''O''-neoh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. The most common industrial diol is ethylene glycol. Examples of diols in which the hydroxyl functional groups are more widely separated include 1,4-butanediol and propylene-1,3-diol, or beta propylene glycol, . Synthesis of classes of diols Geminal diols A geminal diol has two hydroxyl groups bonded to the same atom. These species arise by hydration of the carbonyl compounds. The hydration is usually unfavorable, but a notable exception is formaldehyde which, in water, exists in equilibrium with methanediol H2C(OH)2. Another example is (F3C)2C(OH)2, the hydrated form of hexafluoroacetone. Many gem-diols undergo further condensation to give dimeric and oligomeric derivatives. This reaction applies to glyoxal and related aldehydes. Vicinal diols In a vicinal diol, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known as glycerides. Because it has antimicrobial and antiviral properties, it is widely used in wound and burn treatments approved by the U.S. Food and Drug Administration. Conversely, it is also used as a bacterial culture medium. It can be used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature. Structure Although achiral, glycerol is prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the stereospecific numbering labels the molecule with a "sn-" prefix before the stem name of the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
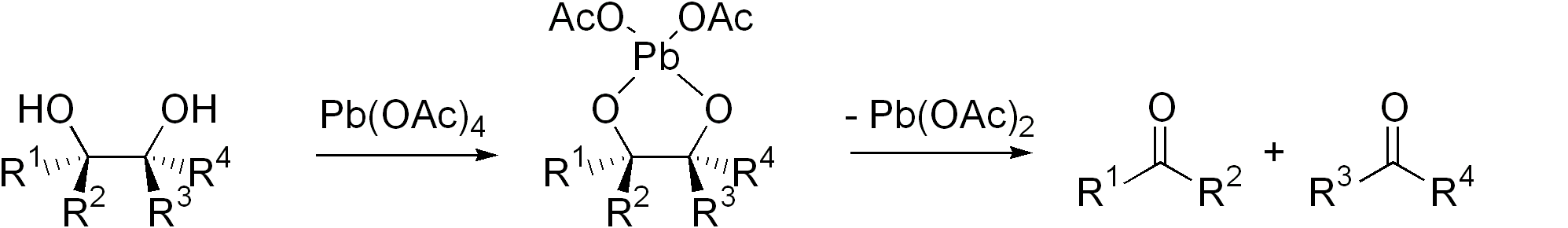
Diol Cleavage
Glycol cleavage is a specific type of organic chemistry oxidation. The carbon–carbon bond in a vicinal diol (glycol) is cleaved and instead the two oxygen atoms become double-bonded to their respective carbon atoms. Depending on the substitution pattern in the diol, these carbonyls can be either ketones or aldehydes. Glycol cleavage is an important reaction in the laboratory because it is useful for determining the structures of sugars. After cleavage takes place the ketone and aldehyde fragments can be inspected and the location of the former hydroxyl groups ascertained. Reagents Periodic acid (HIO4), (diacetoxyiodo)benzene (PhI(OAc)2) and lead tetraacetate (Pb(OAc)4) are the most common reagents used for glycol cleavage, processes called the Malaprade reaction and Criegee oxidation, respectively. These reactions are most efficient when a cyclic intermediate can form, with the iodine or lead atom linking both oxygen atoms. The ring then fragments, with breakage of the carbon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vicinal (chemistry)
In chemistry the descriptor vicinal (from Latin ''vicinus'' = neighbor), abbreviated ''vic'', describes any two functional groups bonded to two adjacent carbon atoms (i.e., in a 1,2-relationship). Relation of atoms in a molecule For example, the molecule 2,3-dibromobutane carries two vicinal bromine atoms and 1,3-dibromobutane does not. Mostly, the use of the term vicinal is restricted to two ''identical'' functional groups. Likewise in a ''gem-''dibromide the prefix ''gem'', an abbreviation of geminal, signals that both bromine atoms are bonded to the ''same'' atom (i.e., in a 1,1-relationship). For example, 1,1-dibromobutane is geminal. While comparatively less common, the term hominal has been suggested as a descriptor for groups in a 1,3-relationship. Like other such descriptors as syn, anti, exo or endo, the description ''vicinal'' helps explain how different parts of a molecule are related to each other either structurally or spatially. The vicinal adjective is sometim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |