|
Reversible Addition−fragmentation Chain-transfer Polymerization
Reversible addition−fragmentation chain-transfer or RAFT polymerization is one of several kinds of reversible-deactivation radical polymerization. It makes use of a chain-transfer agent in the form of a thiocarbonylthio compound (or similar, from here on referred to as a RAFT agent, see Figure 1) to afford control over the generated molecular weight and polydispersity during a free-radical polymerization. Discovered at the Commonwealth Scientific and Industrial Research Organisation (CSIRO) of Australia in 1998, RAFT polymerization is one of several living or controlled radical polymerization techniques, others being atom transfer radical polymerization (ATRP) and nitroxide-mediated polymerization (NMP), etc. RAFT polymerization uses thiocarbonylthio compounds, such as dithioesters, thiocarbamates, and xanthates, to mediate the polymerization via a reversible chain-transfer process. As with other controlled radical polymerization techniques, RAFT polymerizations can be performed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monomer
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization. Classification Monomers can be classified in many ways. They can be subdivided into two broad classes, depending on the kind of the polymer that they form. Monomers that participate in condensation polymerization have a different stoichiometry than monomers that participate in addition polymerization: : Other classifications include: *natural vs synthetic monomers, e.g. glycine vs caprolactam, respectively *polar vs nonpolar monomers, e.g. vinyl acetate vs ethylene, respectively *cyclic vs linear, e.g. ethylene oxide vs ethylene glycol, respectively The polymerization of one kind of monomer gives a homopolymer. Many polymers are copolymers, meaning that they are derived from two different monomers. In the case of condensation polymerizations, the r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azobisisobutyronitrile
Azobisisobutyronitrile (abbreviated AIBN) is an organic compound with the formula CH3)2C(CN)sub>2N2. This white powder is soluble in alcohols and common organic solvents but is insoluble in water. It is often used as a foamer in plastics and rubber and as a radical initiator. As an azo initiator, radicals resulting from AIBN have multiple benefits over common organic peroxides. For example, they do not have oxygenated byproducts or much yellow discoloration. Additionally, they do not cause too much grafting and therefore are often used when making adhesives, acrylic fibers, detergents, etc. Mechanism of decomposition In its most characteristic reaction, AIBN decomposes, eliminating a molecule of nitrogen gas to form two 2-cyanoprop-2-yl radicals: : Because azobisisobutyronitrile readily gives off free radicals, it is often used as a radical initiator. This happens at temperatures above 40 °C, but in experiments is more commonly done at temperatures between 66 °C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radical Initiator
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions. These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical initiators are utilized in industrial processes such as polymer synthesis. Typical examples are molecules with a nitrogen-halogen bond, azo compounds, and organic and inorganic peroxides. Main types of initiation reaction *Halogens undergo homolytic fission relatively easily. Chlorine, for example, gives two chlorine radicals (Cl•) by irradiation with ultraviolet light. This process is used for chlorination of alkanes. *Azo compounds (R- N=N-R') can be the precursor of two carbon-centered radicals (R• and R'•) and nitrogen gas upon heating and/or by irradiation. For example, AIBN and ABCN yield isobutyronitrile and cyclohexanecarbonitrile radicals, respectively. : *Organic peroxides each have a peroxide bond (- O-O-), which is readi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radical Polymerization
In polymer chemistry, free-radical polymerization (FRP) is a method of polymerization by which a polymer forms by the successive addition of free-radical building blocks (repeat units). Free radicals can be formed by a number of different mechanisms, usually involving separate initiator molecules. Following its generation, the initiating free radical adds (nonradical) monomer units, thereby growing the polymer chain. Free-radical polymerization is a key synthesis route for obtaining a wide variety of different polymers and materials composites. The relatively non-specific nature of free-radical chemical interactions makes this one of the most versatile forms of polymerization available and allows facile reactions of polymeric free-radical chain ends and other chemicals or substrates. In 2001, 40 billion of the 110 billion pounds of polymers produced in the United States were produced by free-radical polymerization. Free-radical polymerization is a type of chain-growth polymeriz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Living Polymerization
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is also much larger than the rate of chain propagation. The result is that the polymer chains grow at a more constant rate than seen in traditional chain polymerization and their lengths remain very similar (i.e. they have a very low polydispersity index). Living polymerization is a popular method for synthesizing block copolymers since the polymer can be synthesized in stages, each stage containing a different monomer. Additional advantages are predetermined molar mass and control over end-groups. Living polymerization is desirable because it offers precision and control in macromolecular synthesis. This is important since many of the novel/useful properties of polymers result from t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RAFT Mechanism
A raft is any flat structure for support or transportation over water. It is usually of basic design, characterized by the absence of a hull. Rafts are usually kept afloat by using any combination of buoyant materials such as wood, sealed barrels, or inflated air chambers (such as pontoons), and are typically not propelled by an engine. Rafts are an ancient mode of transport; naturally-occurring rafts such as entwined vegetation and pieces of wood have been used to traverse water since the dawn of humanity. Human-made rafts Traditional or primitive rafts were constructed of wood or reeds. Modern rafts may also use pontoons, drums, or extruded polystyrene blocks. Inflatable rafts up to the 20th century used flotation chambers made of goat- or buffalo-skins, but most now use durable, multi-layered rubberized fabrics. Depending on its use and size, it may have a superstructure, masts, or rudders. Timber rafting is used by the logging industry for the transportation of logs, b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Products Of A RAFT Polymerisation
Product may refer to: Business * Product (business), an item that serves as a solution to a specific consumer problem. * Product (project management), a deliverable or set of deliverables that contribute to a business solution Mathematics * Product (mathematics) Algebra * Direct product Set theory * Cartesian product of sets Group theory * Direct product of groups * Semidirect product * Product of group subsets * Wreath product * Free product * Zappa–Szép product (or knit product), a generalization of the direct and semidirect products Ring theory * Product of rings * Ideal operations, for product of ideals Linear algebra * Scalar multiplication * Matrix multiplication * Inner product, on an inner product space * Exterior product or wedge product * Multiplication of vectors: ** Dot product ** Cross product ** Seven-dimensional cross product ** Triple product, in vector calculus * Tensor product Topology * Product topology Algebraic topology * Cap product * Cup pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom Transfer Radical Polymerization
Atom transfer radical polymerization (ATRP) is an example of a reversible-deactivation radical polymerization. Like its counterpart, ATRA, or atom transfer radical addition, ATRP is a means of forming a carbon-carbon bond with a transition metal catalyst. Polymerization from this method is called atom transfer radical addition polymerization (ATRAP). As the name implies, the atom transfer step is crucial in the reaction responsible for uniform polymer chain growth. ATRP (or transition metal-mediated living radical polymerization) was independently discovered by Mitsuo Sawamoto and by Krzysztof Matyjaszewski and Jin-Shan Wang in 1995. ::The following scheme presents a typical ATRP reaction: Overview of ATRP ATRP usually employs a transition metal complex as the catalyst with an alkyl halide as the initiator (R-X). Various transition metal complexes, namely those of Cu, Fe, Ru, Ni, and Os, have been employed as catalysts for ATRP. In an ATRP process, the dormant species is activated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
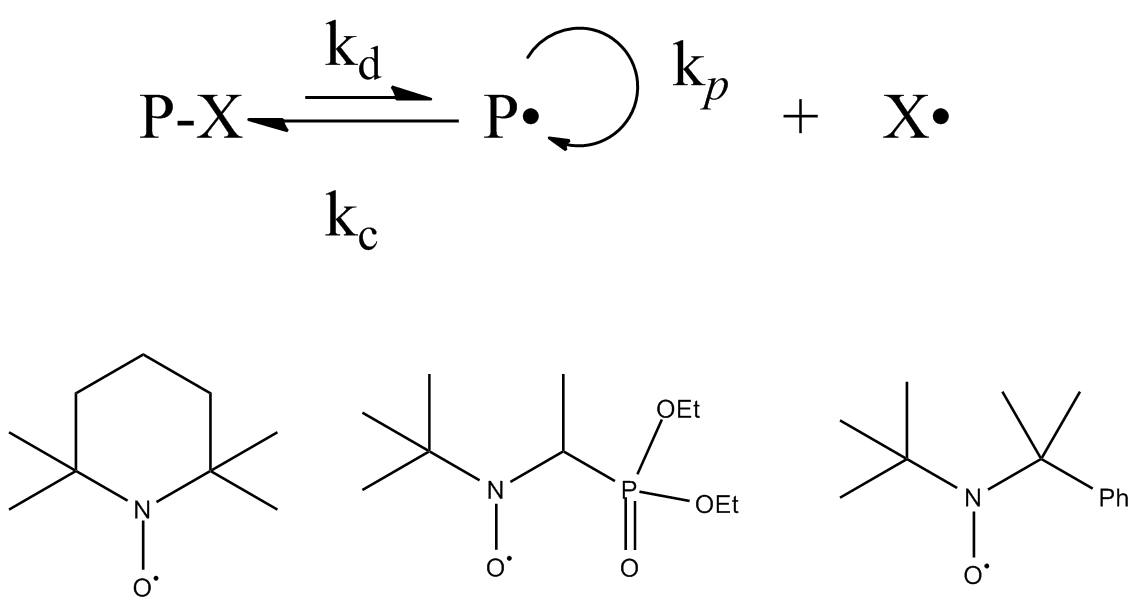
Controlled Radical Polymerization
Living free radical polymerization is a type of living polymerization where the active polymer chain end is a free radical. Several methods exist. IUPAC recommends to use the term "reversible-deactivation radical polymerization" instead of "living free radical polymerization", though the two terms are not synonymous. Reversible-deactivation polymerization There is a mode of polymerization referred to as reversible-deactivation polymerization which is distinct from living polymerization, despite some common features. Living polymerization requires a complete absence of termination reactions, whereas reversible-deactivation polymerization may contain a similar fraction of termination as conventional polymerization with the same concentration of active species. Some important aspects of these are compared in the table: Catalytic chain transfer and cobalt mediated radical polymerization Catalytic chain transfer polymerization is not a strictly living form of polymerization. Yet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyacrylic Acid
Poly(acrylic acid) (PAA; trade name Carbomer) is a polymer with the formula (CH2-CHCO2H)n. It is a derivative of acrylic acid (CH2=CHCO2H). In addition to the homopolymers, a variety of copolymers and crosslinked polymers, and partially deprotonated derivatives thereof are known and of commercial value. In a water solution at neutral pH, PAA is an anionic polymer, i.e., many of the side chains of PAA lose their protons and acquire a negative charge. Partially or wholly deprotonated PAAs are polyelectrolytes, with the ability to absorb and retain water and swell to many times their original volume. These properties – acid-base and water-attracting – are the bases of many applications. = Synthesis = PAA is produced by free radical polymerization. Initiators include potassium persulfate and AIBN. Polyacrylic acid is a polyolefin. It can be viewed as polyethylene with carboxylic acid (CO2H) substituents on alternating carbons. Owing to these groups, alternating carbon atoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_Reaction.png)