|
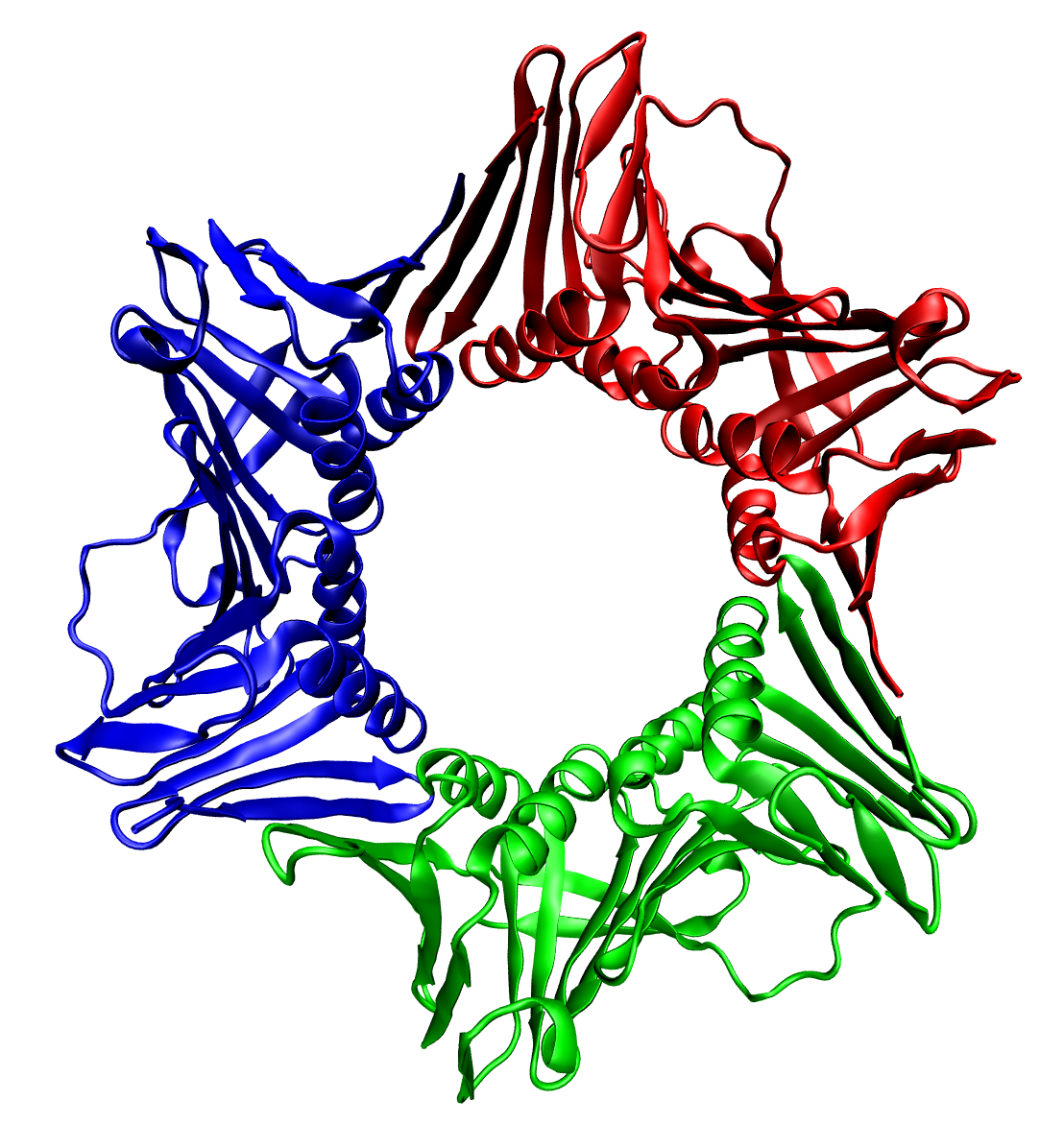
Respirasome
Modern biological research has revealed strong evidence that the enzymes of the mitochondrial respiratory chain assemble into larger, supramolecular structures called supercomplexes, instead of the traditional fluid model of discrete enzymes dispersed in the inner mitochondrial membrane. These supercomplexes are functionally active and necessary for forming stable respiratory complexes. One supercomplex of complex I, III, and IV make up a unit known as a respirasome. Respirasomes have been found in a variety of species and tissues, including rat brain, liver, kidney, skeletal muscle, heart, bovine heart, human skin fibroblasts, fungi, plants, and C. elegans. History In 1955, biologists Britton Chance and G. R. Williams were the first to propose the idea that respiratory enzymes assemble into larger complexes, although the fluid state model remained the standard. However, as early as 1985, researchers had begun isolating Complex III/Complex IV supercomplexes from bacteria an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Respirasome Dudkina 2011
Modern biological research has revealed strong evidence that the enzymes of the mitochondrial respiratory chain assemble into larger, supramolecular structures called supercomplexes, instead of the traditional fluid model of discrete enzymes dispersed in the inner mitochondrial membrane. These supercomplexes are functionally active and necessary for forming stable respiratory complexes. One supercomplex of complex I, III, and IV make up a unit known as a respirasome. Respirasomes have been found in a variety of species and tissues, including rat brain, liver, kidney, skeletal muscle, heart, bovine heart, human skin fibroblasts, fungi, plants, and C. elegans. History In 1955, biologists Britton Chance and G. R. Williams were the first to propose the idea that respiratory enzymes assemble into larger complexes, although the fluid state model remained the standard. However, as early as 1985, researchers had begun isolating Complex III/Complex IV supercomplexes from bacteria an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gel Electrophoresis Of Proteins
Protein electrophoresis is a method for analysing the proteins in a fluid or an extract. The electrophoresis may be performed with a small volume of sample in a number of alternative ways with or without a supporting medium: SDS polyacrylamide gel electrophoresis (in short: gel electrophoresis, PAGE, or SDS-electrophoresis), free-flow electrophoresis, electrofocusing, isotachophoresis, affinity electrophoresis, immunoelectrophoresis, counterelectrophoresis, and capillary electrophoresis. Each method has many variations with individual advantages and limitations. Gel electrophoresis is often performed in combination with electroblotting immunoblotting to give additional information about a specific protein. Because of practical limitations, protein electrophoresis is generally not suited as a preparative method. Denaturing gel methods SDS-PAGE SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis, describes a collection of related techniques to separate proteins ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saccharomyces Cerevisiae
''Saccharomyces cerevisiae'' () (brewer's yeast or baker's yeast) is a species of yeast (single-celled fungus microorganisms). The species has been instrumental in winemaking, baking, and brewing since ancient times. It is believed to have been originally isolated from the skin of grapes. It is one of the most intensively studied eukaryotic model organisms in molecular biology, molecular and cell biology, much like ''Escherichia coli'' as the model bacteria, bacterium. It is the microorganism behind the most common type of fermentation (biochemistry), fermentation. ''S. cerevisiae'' cells are round to ovoid, 5–10 micrometre, μm in diameter. It reproduces by budding. Many proteins important in human biology were first discovered by studying their Homology (biology), homologs in yeast; these proteins include cell cycle proteins, signaling proteins, and protein-processing enzymes. ''S. cerevisiae'' is currently the only yeast cell known to have Berkeley body, Berkeley bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrameric Protein
A tetrameric protein is a protein with a quaternary structure of four subunits (tetrameric). Homotetramers have four identical subunits (such as glutathione S-transferase), and heterotetramers are complexes of different subunits. A tetramer can be assembled as dimer of dimers with two homodimer subunits (such as sorbitol dehydrogenase), or two heterodimer subunits (such as hemoglobin). Subunit interactions in tetramers The interactions between subunits forming a tetramer is primarily determined by non covalent interaction. Hydrophobic effects, hydrogen bonds and electrostatic interactions are the primary sources for this binding process between subunits. For homotetrameric proteins such as Sorbitol dehydrogenase (SDH), the structure is believed to have evolved going from a monomeric to a dimeric and finally a tetrameric structure in evolution. The binding process in SDH and many other tetrameric enzymes can be described by the gain in free energy which can be determined from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Trimer
In biochemistry, a protein trimer is a macromolecular complex formed by three, usually non-covalently bound, macromolecules like proteins or nucleic acids. A homotrimer would be formed by three identical molecules. A heterotrimer would be formed by three different macromolecules. Type II Collagen is an example of homotrimeric protein. Porins usually arrange themselves in membranes as trimers. Bacteriophage T4 tail fiber Multiple copies of a polypeptide encoded by a gene often can form an aggregate referred to as a multimer. When a multimer is formed from polypeptides produced by two different mutant alleles of a particular gene, the mixed multimer may exhibit greater functional activity than the unmixed multimers formed by each of the mutants alone. When a mixed multimer displays increased functionality relative to the unmixed multimers, the phenomenon is referred to as intragenic complementation. The distal portion of each of the bacteriophage T4 tail fibers is encoded by ge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufer aft ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain lysyl ((CH2)4NH2), classifying it as a basic, charged (at physiological pH), aliphatic amino acid. It is encoded by the codons AAA and AAG. Like almost all other amino acids, the α-carbon is chiral and lysine may refer to either enantiomer or a racemic mixture of both. For the purpose of this article, lysine will refer to the biologically active enantiomer L-lysine, where the α-carbon is in the ''S'' configuration. The human body cannot synthesize lysine. It is essential in humans and must therefore be obtained from the diet. In organisms that synthesise lysine, two main biosynthetic pathways exist, the diaminopimelate and α-aminoadipate pathways, which employ distinct e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutralization (chemistry)
In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react quantitatively with each other. In a reaction in water, neutralization results in there being no excess of hydrogen or hydroxide ions present in the solution. The pH of the neutralized solution depends on the acid strength of the reactants. Meaning of "neutralization" In the context of a chemical reaction the term neutralization is used for a reaction between an acid and a base or alkali. Historically, this reaction was represented as :acid + base (alkali) → salt + water For example: :HCl + NaOH → NaCl + H2O The statement is still valid as long as it is understood that in an aqueous solution the substances involved are subject to dissociation, which changes the ionization state of the substances. The arrow sign, →, is used because the reaction is complete, that is, neutralization is a quantitative reaction. A more general definition is base ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cardiolipin
Cardiolipin (IUPAC name 1,3-bis(''sn''-3’-phosphatidyl)-''sn''-glycerol) is an important component of the inner mitochondrial membrane, where it constitutes about 20% of the total lipid composition. It can also be found in the membranes of most bacteria. The name "cardiolipin" is derived from the fact that it was first found in animal hearts. It was first isolated from beef heart in the early 1940s by Mary C. Pangburn. In mammalian cells, but also in plant cells, cardiolipin (CL) is found almost exclusively in the inner mitochondrial membrane, where it is essential for the optimal function of numerous enzymes that are involved in mitochondrial energy metabolism. Structure Cardiolipin (CL) is a kind of diphosphatidylglycerol lipid. Two phosphatidic acid moieties connect with a glycerol backbone in the center to form a dimeric structure. So it has four alkyl groups and potentially carries two negative charges. As there are four distinct alkyl chains in cardiolipin, the potenti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid
Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries, and in nanotechnology. Lipids may be broadly defined as hydrophobic or amphiphilic small molecules; the amphiphilic nature of some lipids allows them to form structures such as vesicles, multilamellar/unilamellar liposomes, or membranes in an aqueous environment. Biological lipids originate entirely or in part from two distinct types of biochemical subunits or "building-blocks": ketoacyl and isoprene groups. Using this approach, lipids may be divided into eight categories: fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, saccharolipids, and polyketides (derived from condensati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ATPase
ATPases (, Adenosine 5'-TriPhosphatase, adenylpyrophosphatase, ATP monophosphatase, triphosphatase, SV40 T-antigen, ATP hydrolase, complex V (mitochondrial electron transport), (Ca2+ + Mg2+)-ATPase, HCO3−-ATPase, adenosine triphosphatase) are a class of enzymes that catalyze the decomposition of ATP into ADP and a free phosphate ion or the inverse reaction. This dephosphorylation reaction releases energy, which the enzyme (in most cases) harnesses to drive other chemical reactions that would not otherwise occur. This process is widely used in all known forms of life. Some such enzymes are integral membrane proteins (anchored within biological membranes), and move solutes across the membrane, typically against their concentration gradient. These are called transmembrane ATPases. Functions Transmembrane ATPases import metabolites necessary for cell metabolism and export toxins, wastes, and solutes that can hinder cellular processes. An important example is the sodium-potass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Complex II
Succinate dehydrogenase (SDH) or succinate-coenzyme Q reductase (SQR) or respiratory complex II is an enzyme complex, found in many bacterial Cell (biology), cells and in the inner mitochondrial membrane of eukaryotes. It is the only enzyme that participates in both the citric acid cycle and the electron transport chain. Histochemical analysis showing high succinate dehydrogenase in muscle demonstrates high mitochondrial content and high oxidative potential. In step 6 of the citric acid cycle, SQR catalyzes the oxidation of succinate to fumarate with the redox, reduction of ubiquinone to ubiquinol. This occurs in the inner mitochondrial biological membrane, membrane by coupling the two reactions together. Structure Subunits Mitochondrion, Mitochondrial and many bacterial SQRs are composed of four structurally different Protein subunit, subunits: two hydrophilic and two hydrophobic. The first two subunits, a flavoprotein (SdhA) and an iron-sulfur protein (SdhB), form a hyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |