|
Recarbrio
Imipenem/cilastatin/relebactam, sold under the brand name Recarbrio, is a fixed-dose combination medication used as an antibiotic. In 2019, it was approved for use in the United States for the treatment of complicated urinary tract and complicated intra-abdominal infections. It is administered via intravenous injection. The most common adverse reactions include nausea, diarrhea, headache, fever and increased liver enzymes. The most common adverse reactions observed in people treated for hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP) include increased aspartate/ alanine aminotransferases (increased liver enzymes), anemia, diarrhea, hypokalemia (low potassium), and hyponatremia (low sodium). Medical uses In the United States imipenem/cilastatin/relebactam is indicated In medicine, an indication is a valid reason to use a certain test, medication, procedure, or surgery. There can be multiple indications to use a procedur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
β-Lactam Antibiotic
β-lactam antibiotics (beta-lactam antibiotics) are antibiotics that contain a beta-lactam ring in their chemical structure. This includes penicillin derivatives (penams), cephalosporins and cephamycins (cephems), monobactams, carbapenems and carbacephems. Most β-lactam antibiotics work by inhibiting cell wall biosynthesis in the bacterial organism and are the most widely used group of antibiotics. Until 2003, when measured by sales, more than half of all commercially available antibiotics in use were β-lactam compounds. The first β-lactam antibiotic discovered, penicillin, was isolated from a rare variant of ''Penicillium notatum'' (since renamed ''Penicillium chrysogenum)''. Bacteria often develop resistance to β-lactam antibiotics by synthesizing a β-lactamase, an enzyme that attacks the β-lactam ring. To overcome this resistance, β-lactam antibiotics can be given with β-lactamase inhibitors such as clavulanic acid. Medical use β-lactam antibiotics are indicated for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
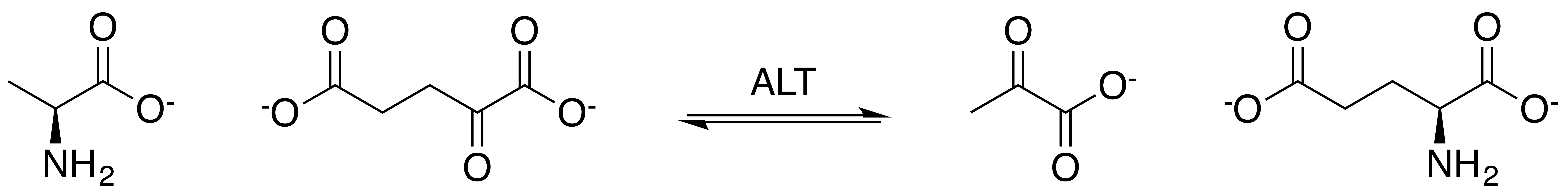
Alanine Aminotransferase
Alanine transaminase (ALT) is a transaminase enzyme (). It is also called alanine aminotransferase (ALT or ALAT) and was formerly called serum glutamate-pyruvate transaminase or serum glutamic-pyruvic transaminase (SGPT) and was first characterized in the mid-1950s by Arthur Karmen and colleagues. ALT is found in plasma and in various body tissues but is most common in the liver. It catalyzes the two parts of the alanine cycle. Serum ALT level, serum AST (aspartate transaminase) level, and their ratio (AST/ALT ratio) are commonly measured clinically as biomarkers for liver health. The tests are part of blood panels. The half-life of ALT in the circulation approximates 47 hours. Aminotransferase is cleared by sinusoidal cells in the liver. Function ALT catalyzes the transfer of an amino group from L-alanine to α-ketoglutarate, the products of this reversible transamination reaction being pyruvate and L-glutamate. : L-alanine + α-ketoglutarate ⇌ pyruvate + L-glutamate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Combination Antibiotics
In mathematics, a combination is a selection of items from a set that has distinct members, such that the order of selection does not matter (unlike permutations). For example, given three fruits, say an apple, an orange and a pear, there are three combinations of two that can be drawn from this set: an apple and a pear; an apple and an orange; or a pear and an orange. More formally, a ''k''-combination of a set ''S'' is a subset of ''k'' distinct elements of ''S''. So, two combinations are identical if and only if each combination has the same members. (The arrangement of the members in each set does not matter.) If the set has ''n'' elements, the number of ''k''-combinations, denoted as C^n_k, is equal to the binomial coefficient \binom nk = \frac, which can be written using factorials as \textstyle\frac whenever k\leq n, and which is zero when k>n. This formula can be derived from the fact that each ''k''-combination of a set ''S'' of ''n'' members has k! permutations so P^n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbapenem Antibiotics
Carbapenems are a class of very effective antibiotic agents most commonly used for the treatment of severe bacterial infections. This class of antibiotics is usually reserved for known or suspected multiple drug resistance, multidrug-resistant (MDR) bacterial infections. Similar to penicillins and cephalosporins, carbapenems are members of the beta lactam class of antibiotics, which kill bacteria by binding to penicillin-binding proteins, thus inhibiting bacterial cell wall synthesis. However, these agents individually exhibit a broader spectrum of activity compared to most cephalosporins and penicillins. Furthermore, carbapenems are typically unaffected by emerging antimicrobial resistance, antibiotic resistance, even to other beta-lactams. Carbapenem antibiotics were originally developed at Merck & Co. from the carbapenem thienamycin, a naturally derived product of ''Streptomyces cattleya''. Concern has arisen in recent years over increasing rates of resistance to carbapenems, as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imipenem/cilastatin
Imipenem/cilastatin, sold under the brand name Primaxin among others, is an antibiotic useful for the treatment of a number of bacterial infections. It is made from a combination of imipenem and cilastatin. Specifically it is used for pneumonia, sepsis, endocarditis, joint infections, intra-abdominal infections, and urinary tract infections. It is given by injection into a vein or muscle. Common side effects include nausea, diarrhea, and pain at the site of injection. Other side effects may include ''Clostridium difficile'' diarrhea and allergic reactions including anaphylaxis. It is unclear if use during pregnancy is safe for the baby. Imipenem is in the carbapenem family of medications and works by interfering with the bacteria's cell wall. Cilastatin blocks the activity of dehydropeptidase I which prevents the breakdown of imipenem. Imipenem/cilastatin was first sold in 1987. It is on the World Health Organization's List of Essential Medicines. Medical uses Imipenem/ci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Priority Review
Priority review is a program of the United States Food and Drug Administration (FDA) to expedite the review process for drugs that are expected to have a particularly great impact on the treatment of a disease. The priority review voucher program is a program that grants a voucher for priority review to a drug developer as an incentive to develop treatments for disease indications with limited profitability. Priority review vouchers are currently earned by pharmaceutical companies for the development and approval of drugs treating neglected tropical diseases, rare pediatric diseases, and "medical countermeasures" for terrorism. The voucher can be used for future drugs that could have wider indications for use, but the company is required to pay a fee (approximately $2.8 million) to use the voucher. When seeking approval for a drug, manufacturers can apply to the FDA for priority review. This is granted when a drug is intended to treat a serious condition and would "provide a si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fast Track (FDA)
Fast track is a designation by the United States Food and Drug Administration (FDA) of an investigational drug for expedited review to facilitate development of drugs that treat a serious or life-threatening condition and fill an unmet medical need. Fast Track designation must be requested by the drug company. The request can be initiated at any time during the drug development process. FDA will review the request and attempt to make a decision within sixty days. Purpose Fast Track is one of five Food and Drug Administration (FDA) approaches to make new drugs available as rapidly as possible: the others are priority review, breakthrough therapy, accelerated approval and Regenerative Medicine Advanced Therapy. Fast Track was introduced by the FDA Modernization Act of 1997. Requirements Fast track designation is designed to aid in the development and expedite the review of drugs which show promise in treating a serious or life-threatening disease and address an unmet medical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Qualified Infectious Disease Product
The Food and Drug Administration Safety and Innovation Act of 2012 (FDASIA) is a piece of American regulatory legislation signed into law on July 9, 2012. It gives the United States Food and Drug Administration (FDA) the authority to collect user fees from the medical industry to fund reviews of innovator drugs, medical devices, generic drugs and biosimilar biologics. It also creates the breakthrough therapy designation program and extends the priority review voucher program to make eligible rare pediatric diseases. The measure was passed by 96 senators voting for and one voting against. Title I: Fees Relating to Drugs Title I extends through FY2017 the authority of the FDA, through the authority of the Secretary of Health and Human Services, to collect drug application and supplement fees, prescription drug establishment fees, and prescription drug product fees to support the FDA process for reviewing human drug applications. It requires the FDA to submit annually to t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |