|
Range (particle Radiation)
In passing through matter, charged particles ionize and thus lose energy in many steps, until their energy is (almost) zero. The distance to this point is called the range of the particle. The range depends on the type of particle, on its initial energy and on the material through which it passes. For example, if the ionising particle passing through the material is a positive ion like an alpha particle or proton, it will collide with atomic electrons in the material via Coulombic interaction. Since the mass of the proton or alpha particle is much greater than that of the electron, there will be no significant deviation from the radiation's incident path and very little kinetic energy will be lost in each collision. As such, it will take many successive collisions for such heavy ionising radiation to come to a halt within the stopping medium or material. Maximum energy loss will take place in a head-on collision with an electron. Since large angle scattering is rare for positive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charged Particle
In physics, a charged particle is a particle with an electric charge. It may be an ion, such as a molecule or atom with a surplus or deficit of electrons relative to protons. It can also be an electron or a proton, or another elementary particle, which are all believed to have the same charge (except antimatter). Another charged particle may be an atomic nucleus devoid of electrons, such as an alpha particle. A plasma is a collection of charged particles, atomic nuclei and separated electrons, but can also be a gas containing a significant proportion of charged particles. Charges are arbitrarily labeled as ''positive''(+) or ''negative''(-). Only the existence of two 'types' of charges is known, there isn't anything inherent about positive charges that makes them positive, and the same goes for the negative charge. Examples Positively charged particles * protons and atomic nuclei * positrons (antielectrons) * alpha particles * positive charged pions * cation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stopping Power (particle Radiation)
In nuclear and materials physics, stopping power is the retarding force acting on charged particles, typically alpha and beta particles, due to interaction with matter, resulting in loss of particle kinetic energy. Its application is important in areas such as radiation protection, ion implantation and nuclear medicine.ICRU Report 73: Stopping of Ions heavier than Helium, Journal of the ICRU, 5 No. 1 (2005), Oxford Univ. Press Definition and Bragg curve Both charged and uncharged particles lose energy while passing through matter. Positive ions are considered in most cases below. The stopping power depends on the type and energy of the radiation and on the properties of the material it passes. Since the production of an ion pair (usually a positive ion and a (negative) electron) requires a fixed amount of energy (for example, 33.97 eV in dry air), the number of ionizations per path length is proportional to the stopping power. The ''stopping power'' of the material is numeri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Particle Physics
Particle physics or high energy physics is the study of fundamental particles and forces that constitute matter and radiation. The fundamental particles in the universe are classified in the Standard Model as fermions (matter particles) and bosons (force-carrying particles). There are three generations of fermions, but ordinary matter is made only from the first fermion generation. The first generation consists of up and down quarks which form protons and neutrons, and electrons and electron neutrinos. The three fundamental interactions known to be mediated by bosons are electromagnetism, the weak interaction, and the strong interaction. Quarks cannot exist on their own but form hadrons. Hadrons that contain an odd number of quarks are called baryons and those that contain an even number are called mesons. Two baryons, the proton and the neutron, make up most of the mass of ordinary matter. Mesons are unstable and the longest-lived last for only a few hundredt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radiation Length
In physics, the radiation length is a characteristic of a material, related to the energy loss of high energy particles electromagnetically interacting with it. Definition In materials of high atomic number (e.g. W, U, Pu) the electrons of energies >~10 MeV predominantly lose energy by bremsstrahlung, and high-energy photons by pair production. The characteristic amount of matter traversed for these related interactions is called the radiation length , usually measured in g·cm−2. It is both the mean distance over which a high-energy electron loses all but of its energy by bremsstrahlung, and of the mean free path for pair production by a high-energy photon. It is also the appropriate length scale for describing high-energy electromagnetic cascades. The radiation length for a given material consisting of a single type of nucleus can be approximated by the following expression: (http://pdg.lbl.gov/) X_0 = 716.4\;\mathrm g\, \mathrm^ \frac = 1433 \;\mathrm g\, \mathrm^ \fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Attenuation Length
In physics, the attenuation length or absorption length is the distance \lambda into a material when the probability has dropped to 1/e that a particle has ''not'' been absorbed. Alternatively, if there is a beam of particles incident on the material, the attenuation length is the distance where the intensity of the beam has dropped to 1/e, or about 63% of the particles have been stopped. Mathematically, the probability of finding a particle at depth ''x'' into the material is calculated by Beer–Lambert law: :P(x) = e^ \!\,. In general \lambda is material and energy dependent. See also * Beer's Law * Mean free path In physics, mean free path is the average distance over which a moving particle (such as an atom, a molecule, or a photon) travels before substantially changing its direction or energy (or, in a specific context, other properties), typically as ... * Attenuation coefficient * Attenuation (electromagnetic radiation) * Radiation length References * * htt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Radiation
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay. There are two forms of beta decay, β− decay and β+ decay, which produce electrons and positrons respectively. Beta particles with an energy of 0.5 MeV have a range of about one metre in air; the distance is dependent on the particle energy. Beta particles are a type of ionizing radiation and for radiation protection purposes are regarded as being more ionising than gamma rays, but less ionising than alpha particles. The higher the ionising effect, the greater the damage to living tissue, but also the lower the penetrating power of the radiation. Beta decay modes β− decay (electron emission) An unstable atomic nucleus with an excess of neutrons may undergo β− decay, where a neutron is converted into a proton, an electron, and an electron antineutrino (the ant ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Particles
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be produced in other ways. Alpha particles are named after the first letter in the Greek alphabet, α. The symbol for the alpha particle is α or α2+. Because they are identical to helium nuclei, they are also sometimes written as or indicating a helium ion with a +2 charge (missing its two electrons). Once the ion gains electrons from its environment, the alpha particle becomes a normal (electrically neutral) helium atom . Alpha particles have a net spin of zero. Due to the mechanism of their production in standard alpha radioactive decay, alpha particles generally have a kinetic energy of about 5 MeV, and a velocity in the vicinity of 4% of the speed of light. (See discussion below for the limits of these figures in alpha decay.) T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
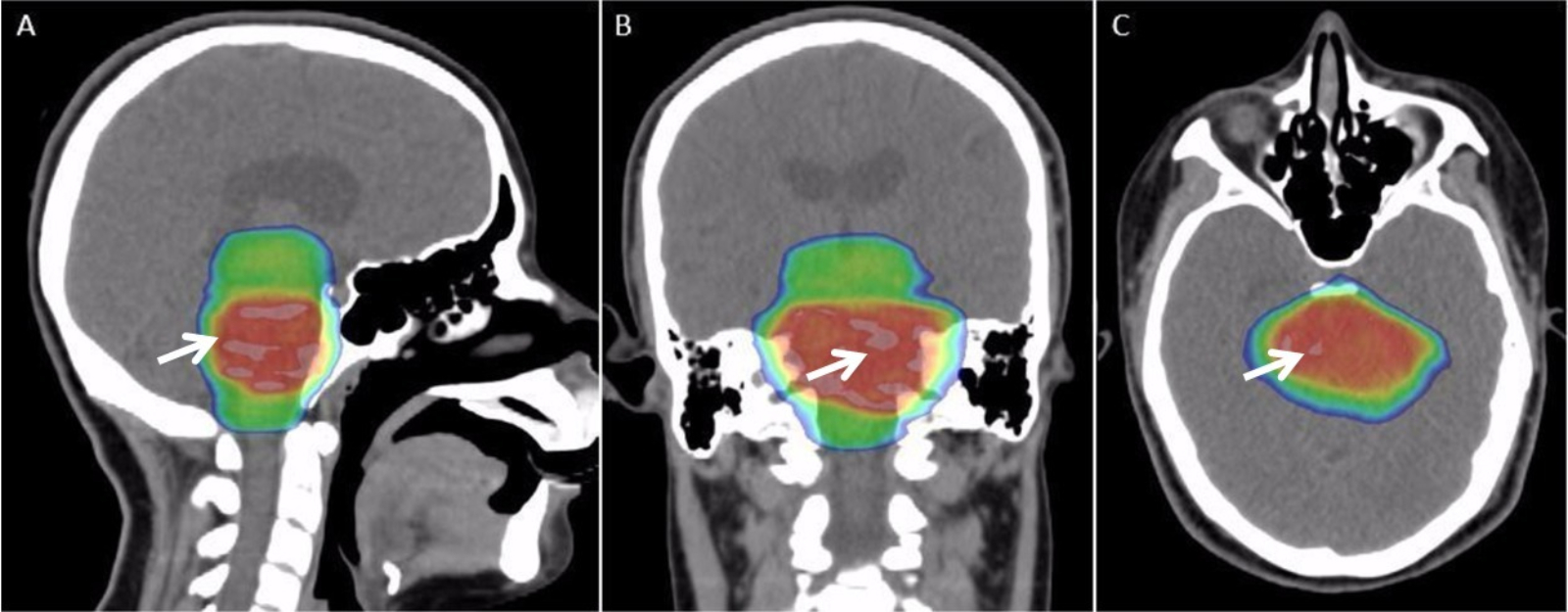
Radiation Therapy
Radiation therapy or radiotherapy, often abbreviated RT, RTx, or XRT, is a therapy using ionizing radiation, generally provided as part of cancer treatment to control or kill malignant cells and normally delivered by a linear accelerator. Radiation therapy may be curative in a number of types of cancer if they are localized to one area of the body. It may also be used as part of adjuvant therapy, to prevent tumor recurrence after surgery to remove a primary malignant tumor (for example, early stages of breast cancer). Radiation therapy is synergistic with chemotherapy, and has been used before, during, and after chemotherapy in susceptible cancers. The subspecialty of oncology concerned with radiotherapy is called radiation oncology. A physician who practices in this subspecialty is a radiation oncologist. Radiation therapy is commonly applied to the cancerous tumor because of its ability to control cell growth. Ionizing radiation works by damaging the DNA of cancerous tissue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bragg Peak
The Bragg peak is a pronounced peak on the Bragg curve which plots the energy loss of ionizing radiation during its travel through matter. For protons, α-rays, and other ion rays, the peak occurs immediately before the particles come to rest. It is named after William Henry Bragg, who discovered it in 1903. When a fast charged particle moves through matter, it ionizes atoms of the material and deposits a dose along its path. A peak occurs because the interaction cross section increases as the charged particle's energy decreases. Energy lost by charged particles is inversely proportional to the square of their velocity, which explains the peak occurring just before the particle comes to a complete stop. In the upper figure, it is the peak for alpha particles of 5.49 MeV moving through air. In the lower figure, it is the narrow peak of the "native" proton beam curve which is produced by a particle accelerator of 250 MeV. The figure also shows the absorption of a beam of ene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
William Henry Bragg
Sir William Henry Bragg (2 July 1862 – 12 March 1942) was an English physicist, chemist, mathematician, and active sportsman who uniquelyThis is still a unique accomplishment, because no other parent-child combination has yet shared a Nobel Prize (in any field). In several cases, a parent has won a Nobel Prize, and then years later, the child has won the Nobel Prize for separate research. An example of this is with Marie Curie and her daughter Irène Joliot-Curie, who are the only mother-daughter pair. Several father-son pairs have won two separate Nobel Prizes. shared a Nobel Prize with his son Lawrence Bragg – the 1915 Nobel Prize in Physics: ''"for their services in the analysis of crystal structure by means of X-rays"''. The mineral Braggite is named after him and his son. He was knighted in 1920. Biography Early years Bragg was born at Westward, near Wigton, Cumberland, England, the son of Robert John Bragg, a merchant marine officer and farmer, and hi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionisation Energy
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected. Uses Everyday examples of gas ionization are such as within a fluorescent lamp or other electrical discharge lamps. It is also used in radiation detectors such as the Geiger-Müller counter or the ionization chamber. The ion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionization
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected. Uses Everyday examples of gas ionization are such as within a fluorescent lamp or other electrical discharge lamps. It is also used in radiation detectors such as the Geiger-Müller counter or the ionization chamber. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |