|
RNF5
E3 ubiquitin-protein ligase RNF5 is an enzyme that in humans is encoded by the ''RNF5'' gene. Function The protein encoded by this gene contains a RING finger, which is a motif known to be involved in protein-protein interactions. This protein is a membrane-bound ubiquitin ligase. It can regulate cell motility by targeting paxillin ubiquitination and altering the distribution and localization of paxillin in cytoplasm and cell focal adhesion In cell biology, focal adhesions (also cell–matrix adhesions or FAs) are large macromolecular assemblies through which mechanical force and regulatory signals are transmitted between the extracellular matrix (ECM) and an interacting cell. More ...s. References Further reading * * * * * * * * * * * * * * External links * RING finger proteins {{protein-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RING Finger Domain
In molecular biology, a RING (short for Really Interesting New Gene) finger domain is a protein structural domain of zinc finger type which contains a C3HC4 amino acid motif which binds two zinc cations (seven cysteines and one histidine arranged non-consecutively). This protein domain contains 40 to 60 amino acids. Many proteins containing a RING finger play a key role in the ubiquitination pathway. Zinc fingers Zinc finger (Znf) domains are relatively small protein motifs that bind one or more zinc atoms, and which usually contain multiple finger-like protrusions that make tandem contacts with their target molecule. They bind DNA, RNA, protein and/or lipid substrates. Their binding properties depend on the amino acid sequence of the finger domains and of the linker between fingers, as well as on the higher-order structures and the number of fingers. Znf domains are often found in clusters, where fingers can have different binding specificities. There are many superfamilies ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene
In biology, the word gene (from , ; "...Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a basic unit of heredity and the molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protein-coding genes and noncoding genes. During gene expression, the DNA is first copied into RNA. The RNA can be directly functional or be the intermediate template for a protein that performs a function. The transmission of genes to an organism's offspring is the basis of the inheritance of phenotypic traits. These genes make up different DNA sequences called genotypes. Genotypes along with environmental and developmental factors determine what the phenotypes will be. Most biological traits are under the influence of polygenes (many different genes) as well as gen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
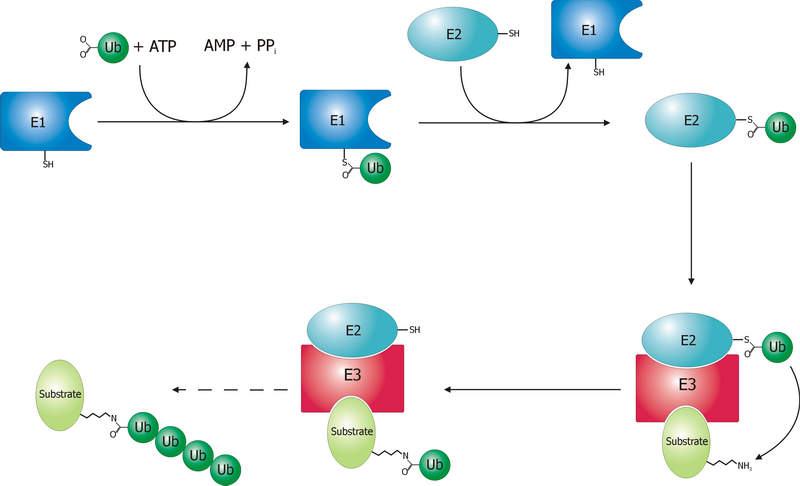
Ubiquitin Ligase
A ubiquitin ligase (also called an E3 ubiquitin ligase) is a protein that recruits an E2 ubiquitin-conjugating enzyme that has been loaded with ubiquitin, recognizes a protein substrate, and assists or directly catalyzes the transfer of ubiquitin from the E2 to the protein substrate. In simple and more general terms, the ligase enables movement of ubiquitin from a ubiquitin carrier to another thing (the substrate) by some mechanism. The ubiquitin, once it reaches its destination, ends up being attached by an isopeptide bond to a lysine residue, which is part of the target protein. E3 ligases interact with both the target protein and the E2 enzyme, and so impart substrate specificity to the E2. Commonly, E3s polyubiquitinate their substrate with Lys48-linked chains of ubiquitin, targeting the substrate for destruction by the proteasome. However, many other types of linkages are possible and alter a protein's activity, interactions, or localization. Ubiquitination by E3 ligases reg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Motility
Motility is the ability of an organism to move independently, using metabolic energy. Definitions Motility, the ability of an organism to move independently, using metabolic energy, can be contrasted with sessility, the state of organisms that do not possess a means of self-locomotion and are normally immobile. Motility differs from mobility, the ability of an object to be moved. The term vagility encompasses both motility and mobility; sessile organisms including plants and fungi often have vagile parts such as fruits, seeds, or spores which may be dispersed by other agents such as wind, water, or other organisms. Motility is genetically determined, but may be affected by environmental factors such as toxins. The nervous system and musculoskeletal system provide the majority of mammalian motility. In addition to animal locomotion, most animals are motile, though some are vagile, described as having passive locomotion. Many bacteria and other microorganisms, and multicellu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paxillin
Paxillin is a protein that in humans is encoded by the ''PXN'' gene. Paxillin is expressed at focal adhesions of non-striated cells and at costameres of striated muscle cells, and it functions to adhere cells to the extracellular matrix. Mutations in ''PXN'' as well as abnormal expression of paxillin protein has been implicated in the progression of various cancers. Structure Human paxillin is 64.5 kDa in molecular weight and 591 amino acids in length. The C-terminal region of paxillin is composed of four tandem double zinc finger LIM domains that are cysteine/histidine-rich with conserved repeats; these serve as binding sites for the protein tyrosine phosphatase-PEST, tubulin and serves as the targeting motif for focal adhesions. The N-terminal region of paxillin has five highly conserved leucine-rich sequences termed LD motifs, which mediate several interactions, including that with pp125FAK and vinculin. The LD motifs are predicted to form amphipathic alpha helices, with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Focal Adhesion
In cell biology, focal adhesions (also cell–matrix adhesions or FAs) are large macromolecular assemblies through which mechanical force and regulatory signals are transmitted between the extracellular matrix (ECM) and an interacting cell. More precisely, focal adhesions are the sub-cellular structures that mediate the regulatory effects (i.e., signaling events) of a cell in response to ECM adhesion. Focal adhesions serve as the mechanical linkages to the ECM, and as a biochemical signaling hub to concentrate and direct numerous signaling proteins at sites of integrin binding and clustering. Structure and function Focal adhesions are integrin-containing, multi-protein structures that form mechanical links between intracellular actin bundles and the extracellular substrate in many cell types. Focal adhesions are large, dynamic protein complexes through which the cytoskeleton of a cell connects to the ECM. They are limited to clearly defined ranges of the cell, at which the pla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |