|
RB-64
RB-64 (22-thiocyanatosalvinorin A) is a semi-synthetic salvinorin A, salvinorin derivative and a κ-opioid receptor (KOR) agonist which is used in scientific research. Its most remarkable property is its functional selectivity for G protein versus Arrestin beta 2, β-arrestin-2. RB-64 has a functional selectivity#bias factor, bias factor of 96 and is analgesic with fewer of the side-effects associated with unbiased KOR agonists. The analgesia-like effect is long-lasting. Compared with unbiased agonists, RB-64 evokes considerably less Receptor-mediated endocytosis, receptor internalization. See also * Herkinorin * Salvinorin B methoxymethyl ether * Salvinorin A * Nalfurafine References Further reading * * Synthetic opioids Kappa-opioid receptor agonists Kappa-opioid receptor antagonists Thiocyanates Biased ligands {{Organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
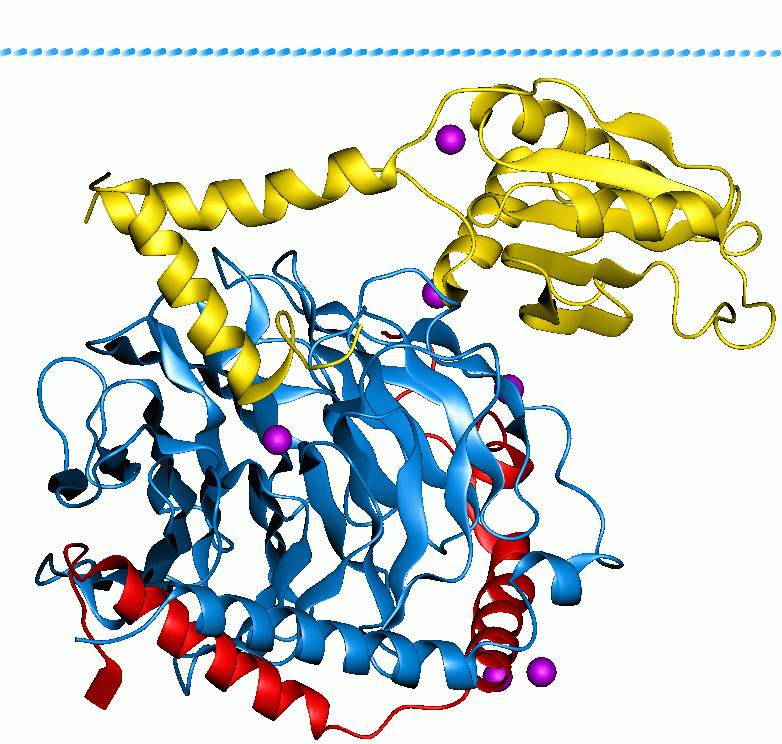
κ-opioid Receptor
The κ-opioid receptor or kappa opioid receptor, abbreviated KOR or KOP, is a G protein-coupled receptor that in humans is encoded by the ''OPRK1'' gene. The KOR is coupled to the G protein Gi/G0 and is one of four related receptors that bind opioid-like compounds in the brain and are responsible for mediating the effects of these compounds. These effects include altering nociception, consciousness, motor control, and mood. Dysregulation of this receptor system has been implicated in alcohol and drug addiction. The KOR is a type of opioid receptor that binds the opioid peptide dynorphin as the primary endogenous ligand (substrate naturally occurring in the body). In addition to dynorphin, a variety of natural alkaloids, terpenes and synthetic ligands bind to the receptor. The KOR may provide a natural addiction control mechanism, and therefore, drugs that target this receptor may have therapeutic potential in the treatment of addiction. There is evidence that distribution ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salvinorin A
Salvinorin A is the main active psychotropic molecule in '' Salvia divinorum''. Salvinorin A is considered a dissociative hallucinogen. It is structurally distinct from other naturally occurring hallucinogens (such as DMT, psilocybin, and mescaline) because it contains no nitrogen atoms; hence, it is not an alkaloid (and cannot be rendered as a salt), but rather is a terpenoid. It also differs in subjective experience, compared to other hallucinogens, and has been described as dissociative. Salvinorin A can produce psychoactive experiences in humans with a typical duration of action being several minutes to an hour or so, depending on the method of ingestion. Salvinorin A is found with several other structurally related salvinorins. Salvinorin is a ''trans''-neoclerodane diterpenoid. It acts as a kappa opioid receptor agonist and is the first known compound acting on this receptor that is not an alkaloid. History Salvinorin A was first described and named in 1982 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salvinorin A
Salvinorin A is the main active psychotropic molecule in '' Salvia divinorum''. Salvinorin A is considered a dissociative hallucinogen. It is structurally distinct from other naturally occurring hallucinogens (such as DMT, psilocybin, and mescaline) because it contains no nitrogen atoms; hence, it is not an alkaloid (and cannot be rendered as a salt), but rather is a terpenoid. It also differs in subjective experience, compared to other hallucinogens, and has been described as dissociative. Salvinorin A can produce psychoactive experiences in humans with a typical duration of action being several minutes to an hour or so, depending on the method of ingestion. Salvinorin A is found with several other structurally related salvinorins. Salvinorin is a ''trans''-neoclerodane diterpenoid. It acts as a kappa opioid receptor agonist and is the first known compound acting on this receptor that is not an alkaloid. History Salvinorin A was first described and named in 1982 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Herkinorin
Herkinorin is an opioid analgesic that is an analog (chemistry), analogue of the natural product salvinorin A. It was discovered in 2005 during structure-activity relationship studies into neoclerodane diterpenes, the family of chemical compounds of which salvinorin A is a member. Unlike salvinorin A, which is a selective κ-opioid receptor agonist with no significant μ-opioid receptor affinity, herkinorin is predominantly a μ-opioid receptor agonist. Compared to salvinorin A, herkinorin has 47× lower Ligand_(biochemistry)#Receptor/ligand_binding_affinity, affinity for κ-opioid receptors (''K''i = 90 nM vs ''K''i = 1.9 nM), and at least 25× higher affinity for μ-opioid receptors (''K''i = 12 nM vs ''K''i > 1000 nM), where it acts as a full agonist (IC50 = 0.5 μM, Emax = 130% vs DAMGO). Herkinorin is a semi-synthetic compound, made from salvinorin B, which is most conveniently made from salvinorin A by deacetylation, since, while both salvinorin A and salvinorin B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salvinorin B Methoxymethyl Ether
Salvinorin B methoxymethyl ether (2-''O''-methoxymethylsalvinorin B) is a semi-synthetic analogue of the natural product salvinorin A used in scientific research. It has a longer duration of action of around 2–3 hours, compared to less than 30 minutes for salvinorin A, and has increased affinity and potency at the κ-opioid receptor. It is made from salvinorin B, which is most conveniently made from salvinorin A by deacetylation. The crystal structure reveals that the methoxy group overlaps with the acetyl group of salvinorin A, but with a different orientation. Salvinorin B methoxymethyl ether has a Ki of 0.60 nM at the κ opioid receptor, and is around five times more potent than salvinorin A in animal studies, although it is still only half as potent as its stronger homolog salvinorin B ethoxymethyl ether (symmetry). See also * Salvinorin B ethoxymethyl ether * RB-64 RB-64 (22-thiocyanatosalvinorin A) is a semi-synthetic salvinorin derivative and a κ-opioid rece ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nalfurafine
Nalfurafine (INN, USAN) (brand name Remitch; former developmental code names TRK-820, AC-820, MT-9938) is an antipruritic (anti-itch drug) that is marketed in Japan for the treatment of uremic pruritus in individuals with chronic kidney disease undergoing hemodialysis. It acts as a potent, selective, centrally-penetrant κ-opioid receptor (KOR) agonist, and is the first and currently the only selective KOR agonist to have been approved for clinical use. It has also been dubiously referred to as the "first non-narcotic opioid drug" in history. History Nalfurafine was derived from structural modification of the opioid antagonist naltrexone. It was first synthesized and characterized in 1998, and was approved for clinical use in Japan as an intravenous drug under the brand name Remitch in 2009. The developer of nalfurafine also sought approval in Europe under the brand name Winfuran, but the Marketing Authorization Application was declined by the European Medicines Agency. The drug w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist. Etymology From the Greek αγωνιστής (agōnistēs), contestant; champion; rival < αγων (agōn), contest, combat; exertion, struggle < αγω (agō), I lead, lead towards, conduct; drive Types of agonists can be activated by either endogenous agonists (such as |
Functional Selectivity
Functional selectivity (or “agonist trafficking”, “biased agonism”, “biased signaling”, "ligand bias" and “differential engagement”) is the ligand-dependent selectivity for certain signal transduction pathways relative to a reference ligand (often the endogenous hormone or peptide) at the same receptor. Functional selectivity can be present when a receptor has several possible signal transduction pathways. To which degree each pathway is activated thus depends on which ligand binds to the receptor. Functional selectivity, or biased signaling, is most extensively characterized at G protein coupled receptors (GPCRs). A number of biased agonists, such as those at muscarinic M2 receptors tested as analgesics or antiproliferative drugs, or those at opioid receptors that mediate pain, show potential at various receptor families to increase beneficial properties while reducing side effects. For example, pre-clinical studies with G protein biased agonists at the μ-opioid re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G Protein
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases. There are two classes of G proteins. The first function as monomeric small GTPases (small G-proteins), while the second function as heterotrimeric G protein complexes. The latter class of complexes is made up of '' alpha'' (α), ''beta'' (β) and ''gamma'' (γ) subunits. In addition, the beta and gamma subunits can form a stable dimeric complex referred to as the beta-gamma complex . Heterotrimeric G proteins located within the cell are activ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arrestin Beta 2
Beta-arrestin-2, also known as arrestin beta-2, is an intracellular protein that in humans is encoded by the ''ARRB2'' gene. Members of arrestin/beta-arrestin protein family are thought to participate in agonist-mediated desensitization of G protein-coupled receptors and cause specific dampening of cellular responses to stimuli such as hormones, neurotransmitters, or sensory signals, as well as having signalling roles in their own right. Arrestin beta 2, like arrestin beta 1, was shown to inhibit beta-adrenergic receptor function in vitro. It is expressed at high levels in the central nervous system and may play a role in the regulation of synaptic receptors. Besides the brain, a cDNA for arrestin beta 2 was isolated from thyroid gland, and thus it may also be involved in hormone-specific desensitization of TSH receptors. Multiple alternatively spliced transcript variants have been found for this gene, but the full-length nature of some variants has not been defined. The protein ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analgesic
An analgesic drug, also called simply an analgesic (American English), analgaesic (British English), pain reliever, or painkiller, is any member of the group of drugs used to achieve relief from pain (that is, analgesia or pain management). It is typically used to induce cooperation with a medical procedure. Analgesics are conceptually distinct from anesthetics, which temporarily reduce, and in some instances eliminate, sensation, although analgesia and anesthesia are neurophysiologically overlapping and thus various drugs have both analgesic and anesthetic effects. Analgesic choice is also determined by the type of pain: For neuropathic pain, traditional analgesics are less effective, and there is often benefit from classes of drugs that are not normally considered analgesics, such as tricyclic antidepressants and anticonvulsants. Various analgesics, such as many NSAIDs, are available over the counter in most countries, whereas various others are prescription drugs owing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor-mediated Endocytosis
Receptor-mediated endocytosis (RME), also called clathrin-mediated endocytosis, is a process by which cells absorb metabolites, hormones, proteins – and in some cases viruses – by the inward budding of the plasma membrane (invagination). This process forms vesicles containing the absorbed substances and is strictly mediated by receptors on the surface of the cell. Only the receptor-specific substances can enter the cell through this process. Process Although receptors and their ligands can be brought into the cell through a few mechanisms (e.g. caveolin and lipid raft), clathrin-mediated endocytosis remains the best studied. Clathrin-mediated endocytosis of many receptor types begins with the ligands binding to receptors on the cell plasma membrane. The ligand and receptor will then recruit adaptor proteins and clathrin triskelions to the plasma membrane around where invagination will take place. Invagination of the plasma membrane then occurs, forming a clathrin-coated pit. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |