|
Rauwolscine 3D
Rauwolscine, also known as isoyohimbine, α-yohimbine, and corynanthidine, is an alkaloid found in various species within the genera ''Rauvolfia'' and '' Corynanthe'' (including ''Pausinystalia''). It is a stereoisomer of yohimbine. Rauwolscine is a central nervous system stimulant, a local anesthetic and a vague aphrodisiac. Rauwolscine acts predominantly as a α2-adrenergic receptor antagonist. It has also been shown to function as a 5-HT1A receptor partial agonist and 5-HT2A and 5-HT2B receptor antagonist. See also * Ajmalicine * Corynanthine * Spegatrine * Yohimbine Yohimbine (), also known as quebrachine, is an indole alkaloid derived from the bark of the African tree '' Pausinystalia johimbe''; also from the bark of the unrelated South American tree ''Aspidosperma quebracho-blanco''. Yohimbine is an α2 ... References {{Serotonergics Indoloquinolizines Tryptamine alkaloids Quinolizidine alkaloids Alkaloids found in Rauvolfia Alpha-2 blockers 5-HT1A ag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rauwolscine 3D
Rauwolscine, also known as isoyohimbine, α-yohimbine, and corynanthidine, is an alkaloid found in various species within the genera ''Rauvolfia'' and '' Corynanthe'' (including ''Pausinystalia''). It is a stereoisomer of yohimbine. Rauwolscine is a central nervous system stimulant, a local anesthetic and a vague aphrodisiac. Rauwolscine acts predominantly as a α2-adrenergic receptor antagonist. It has also been shown to function as a 5-HT1A receptor partial agonist and 5-HT2A and 5-HT2B receptor antagonist. See also * Ajmalicine * Corynanthine * Spegatrine * Yohimbine Yohimbine (), also known as quebrachine, is an indole alkaloid derived from the bark of the African tree '' Pausinystalia johimbe''; also from the bark of the unrelated South American tree ''Aspidosperma quebracho-blanco''. Yohimbine is an α2 ... References {{Serotonergics Indoloquinolizines Tryptamine alkaloids Quinolizidine alkaloids Alkaloids found in Rauvolfia Alpha-2 blockers 5-HT1A ag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT2A Receptor
The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations. 5-HT is short for 5-hydroxy-tryptamine or serotonin. This is the main excitatory receptor subtype among the GPCRs for serotonin, although 5-HT2A may also have an inhibitory effect on certain areas such as the visual cortex and the orbitofrontal cortex. This receptor was first noted for its importance as a target of serotonergic psychedelic drugs such as LSD and psilocybin mushrooms. Later it came back to prominence because it was also found to be mediating, at least partly, the action of many antipsychotic drugs, especially the atypical ones. Downregulation of post-synaptic 5-HT2A receptor is an adaptive process provoked by chronic administration of selective serotonin reuptake inhibitors (SSRIs) and atypical antipsychotics. Suicidal and otherwis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT2A Antagonists
The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations. 5-HT is short for 5-hydroxy-tryptamine or serotonin. This is the main excitatory receptor subtype among the GPCRs for serotonin, although 5-HT2A may also have an inhibitory effect on certain areas such as the visual cortex and the orbitofrontal cortex. This receptor was first noted for its importance as a target of serotonergic psychedelic drugs such as LSD and psilocybin mushrooms. Later it came back to prominence because it was also found to be mediating, at least partly, the action of many antipsychotic drugs, especially the atypical ones. Downregulation of post-synaptic 5-HT2A receptor is an adaptive process provoked by chronic administration of selective serotonin reuptake inhibitors (SSRIs) and atypical antipsychotics. Suicidal and otherwise ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT1A Agonists
The serotonin 1A receptor (or 5-HT1A receptor) is a subtype of serotonin receptor, or 5-HT receptor, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarisation and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene. Distribution The 5-HT1A receptor is the most widespread of all the 5-HT receptors. In the central nervous system, 5-HT1A receptors exist in the cerebral cortex, hippocampus, septum, amygdala, and raphe nucleus in high densities, while low amounts also exist in the basal ganglia and thalamus. The 5-HT1A receptors in the raphe nucleus are largely somatodendritic autoreceptors, whereas those in other areas such as the hippocampus are postsynaptic receptors. Function Neuromodulation 5-HT1A recepto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaloids Found In Rauvolfia
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and, more rarely, other elements such as chlorine, bromine, and phosphorus.Chemical Encyclopedia: alkaloids xumuk.ru Alkaloids are produced by a large variety of organisms including , , |
Quinolizidine Alkaloids
Quinolizidine alkaloids are natural products that have a quinolizidine structure; this includes the lupine alkaloids. Occurrence Quinolizidine alkaloids can be found in the plant family of legumes, especially in papilionaceous plants. While the lupine alkaloids (following their name) can be found in lupines, tinctorin, for example, was isolated from the dyer's broom. Examples More than 200 quinolizidine alkaloids are known which can be classified into 6 structural types: * the lupinine type with 34 known structures, including lupinine and its derivatives * the camoensine type with 6 known structures, including camoensin * the spartein type with 66 structures, including sparteine, lupanine, angustifoline * the α-pyridone type with 25 structures, including anagyrine and cytisine * the matrine type with 31 structures, including matrine * and the ormosanin type with 19 structures, including ormosanine. (–)-Lupinine Structural Formula V2.svg, (–)-lupinine (6R,7S,9S,1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
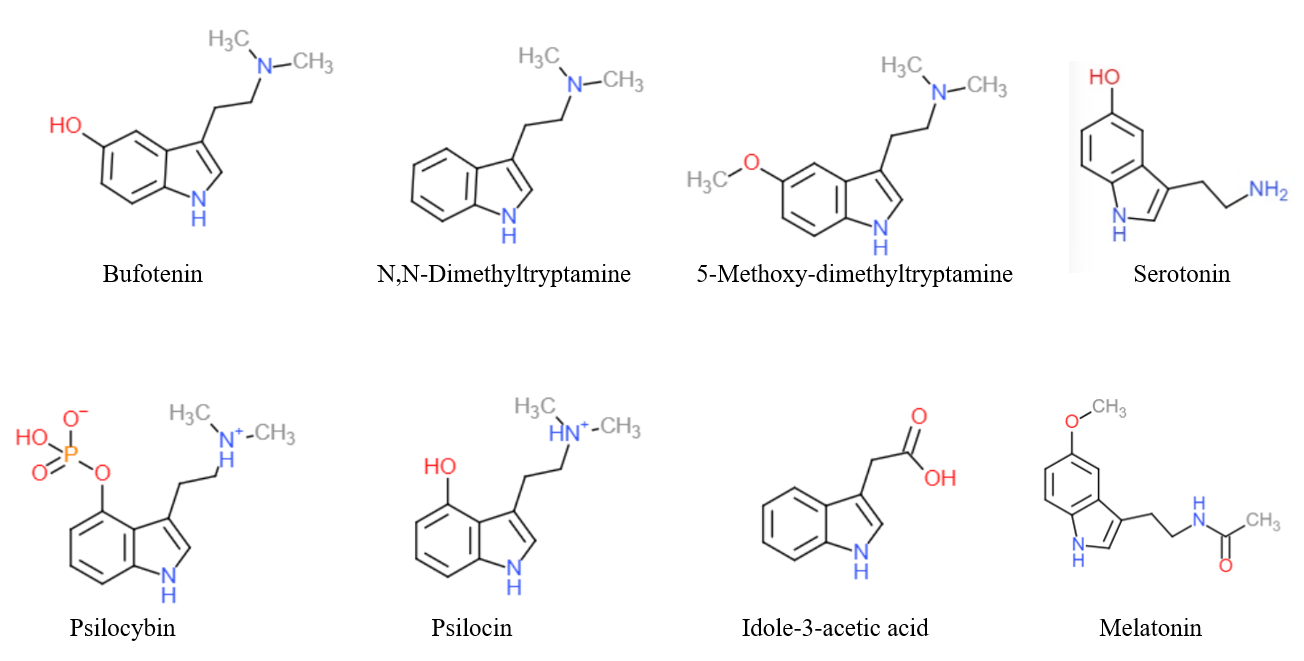
Tryptamine Alkaloids
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the first one being the heterocyclic nitrogen). The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others. Tryptamine has been shown to activate trace amine-associated receptors expressed in the mammalian brain, and regulates the activity of dopaminergic, serotonergic and glutamatergic systems. In the human gut, symbiotic bacteria convert dietary tryptophan to tryptamine, which activates 5-HT4 receptors and regulates gastrointestinal motility. Multiple tryptamine-derived drugs have been developed to treat migraines, while trace amine-associated receptors are being explored as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yohimbine
Yohimbine (), also known as quebrachine, is an indole alkaloid derived from the bark of the African tree ''Pausinystalia johimbe''; also from the bark of the unrelated South American tree ''Aspidosperma quebracho-blanco''. Yohimbine is an α2-adrenergic receptor antagonist, and has been used in a variety of research projects. It is a veterinary drug used to reverse sedation in dogs and deer. While yohimbine behaves as an aphrodisiac in some mammals, it does not do so in humans. It has been prescribed as a treatment for erectile dysfunction, although its reported clinical benefits were modest and it has largely been superseded by the PDE5 inhibitor class of drugs. Substances that have purported to be extracts from the yohimbe tree have been marketed as dietary supplements for various purposes, but they contain highly variable amounts of yohimbine, if any; no published scientific evidence supports their efficacy. Uses Yohimbine is a drug used in veterinary medicine to reverse th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spegatrine
Spegatrine is an α1- and α2-adrenergic receptor antagonist isolated from '' Rauvolfia verticillata''. Its dimer dispegatrine has greater antagonist affinity for α-adrenergic receptors. See also * Ajmalicine * Corynanthine * Rauwolscine * Yohimbine Yohimbine (), also known as quebrachine, is an indole alkaloid derived from the bark of the African tree ''Pausinystalia johimbe''; also from the bark of the unrelated South American tree ''Aspidosperma quebracho-blanco''. Yohimbine is an α2- ... References Alkaloids found in Rauvolfia Alpha-1 blockers Alpha-2 blockers Tryptamine alkaloids Indoloquinolizines {{Organic-chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corynanthine
Corynanthine, also known as rauhimbine, is an alkaloid found in the ''Rauvolfia'' and '' Corynanthe'' (including ''Pausinystalia'') genera of plants. It is one of the two diastereoisomers of yohimbine, the other being rauwolscine. It is also related to ajmalicine. Corynanthine acts as an α1-adrenergic and α2-adrenergic receptor antagonist with approximately 10-fold selectivity for the former site over the latter. This is in contrast to yohimbine and rauwolscine which have around 30-fold higher affinity for the α2-adrenergic receptor over the α1-adrenergic receptor. As a result, corynanthine is not a stimulant (or an aphrodisiac for that matter), but a depressant, and likely plays a role in the antihypertensive properties of ''Rauvolfia'' extracts. Like yohimbine and rauwolscine, corynanthine has also been shown to possess some activity at serotonin receptors. See also * Ajmalicine * Rauwolscine * Spegatrine * Yohimbine Yohimbine (), also known as quebrachine, is an in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |