|
QS21
QS-21 is a purified plant extract used as a vaccine adjuvant. It is derived from the soap bark tree (''Quillaja saponaria''), which is native to the countries of Chile, Peru, and Bolivia. The crude drug Crude drugs are plant or animal drugs that contain natural substances that have undergone only the processes of collection and drying. The term natural substances refers to those substances found in nature that have not had man-made changes made i ... (''Quillajae cortex'') is imported from Peru and Chile. The extract contains water-soluble triterpene glycosides, which are members of a family of plant-based compounds called saponins. It has been tested as an adjuvant in various vaccines in attempts to improve their efficacy. It is believed to enhance both humoral immunity, humoral and cell-mediated immunity. Isolation of QS-21 destroys the soap bark tree, which has resulted in regulation of the tree by the governments where it is grown. A semi-synthesis strategy relies on purify ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agenus
Agenus Inc. is a Lexington, Massachusetts-based biotechnology company focused on immunotherapy including immuno-oncology, a field that uses the immune system to control or cure cancer. The company is developing checkpoint modulators (CPMs), patient-specific anti-cancer vaccines, and adjuvants that can be used with a range of vaccines. CPM development is a particularly fast-moving field, since early products have produced unprecedented clinical benefits for patients. History Agenus (formerly Antigenics Inc.) was founded in 1994 by Garo H. Armen and Pramod K. Srivastava. The company has pioneered immunotherapies, including heat shock protein-based cancer vaccines, a program that has developed into its Prophage Series of personalized anti-cancer vaccines. Antigenics became a public company in February 2000 on the NASDAQ exchange with the ticker symbol AGEN. In 2000 Agenus acquired Aquila Biopharmaceuticals and a year later it acquired Aronex Pharmaceuticals. In February 2014 the com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Soap Bark Tree
''Quillaja saponaria'', the soap bark tree or soapbark, is an evergreen tree in the family Quillajaceae, native to warm temperate central Chile. In Chile it occurs from 32 to 40° South Latitude approximately and at up to 2000 m (6500 ft) above sea level. It can grow to 15–20 m (50–65 ft) in height. The tree has thick, dark bark; smooth, leathery, shiny, oval evergreen leaves 3–5 cm long; white star-shaped flowers 15 mm diameter borne in dense corymbs; and a dry fruit with five follicles each containing 10–20 seeds. Characteristics The inner bark of ''Quillaja saponaria'' can be reduced to powder and employed as a substitute for soap, since it forms a lather with water, owing to the presence of a glycoside saponin, sometimes distinguished as quillaia saponin. It's also applied as an agricultural spray adjuvant. The same, or a closely similar substance, is found in soapwort (''Saponaria officinalis''), in senega root (''Polygala senega'') and in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quillaia
Quillaia is the milled inner bark or small stems and branches of the soapbark ( ''Quillaja saponaria'', Molina). Other names include ''Murillo bark extract'', ''Panama bark extract'', ''Quillaia extract'', ''Quillay bark extract'', and ''Soapbark extract''. Quillaia contains high concentrations of saponins that can be increased further by processing. Highly purified saponins from quillaia are used as adjuvants to enhance the effectiveness of vaccines. Other compounds in the crude extract include tannins and other polyphenols, and calcium oxalate.EFSA Panel on Food Additives and Flavourings (FAF) et alRe-evaluation of Quillaia extract (E 999) as a food additive and safety of the proposed extension of use.EFSA Journal. 06 March 2019. Quillaia is used in the manufacture of food additives, and it is listed as an ingredient in root beer and cream soda. The extract also is used as a humectant in baked goods, frozen dairy products, and puddings and as a foaming agent in soft drinks. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
QS-21
QS-21 is a purified plant extract used as a vaccine adjuvant. It is derived from the soap bark tree (''Quillaja saponaria''), which is native to the countries of Chile, Peru, and Bolivia. The crude drug (''Quillajae cortex'') is imported from Peru and Chile. The extract contains water-soluble triterpene glycosides, which are members of a family of plant-based compounds called saponins. It has been tested as an adjuvant in various vaccines in attempts to improve their efficacy. It is believed to enhance both humoral and cell-mediated immunity. Isolation of QS-21 destroys the soap bark tree, which has resulted in regulation of the tree by the governments where it is grown. A semi-synthesis strategy relies on purifying the prosapogenin (triterpene and branched trisaccharide) part of the molecule and adding the rest of QS-21 synthetically; this is reported to increase the yield by 2 orders of magnitude. This semi-synthetic approach has also facilitated experimentation with alterna ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Defense Production Act Of 1950
The Defense Production Act of 1950 () is a United States federal law enacted on September 8, 1950 in response to the start of the Korean War.Congressional Research ServiceThe Defense Production Act of 1950: History, Authorities, and Considerations for Congress, updated November 20, 2018, accessed January 17, 2019 fas.org It was part of a broad civil defense and war mobilization effort in the context of the Cold War. Its implementing regulations, the Defense Priorities and Allocation System (DPAS), are located at 15 CFR §§700 to 700.93. Since 1950, the Act has been reauthorized over 50 times. It has been periodically amended and remains in force. Provisions The Act currently contains three major sections. The first authorizes the president to require businesses to accept and prioritize contracts for materials deemed necessary for national defense, regardless of a loss incurred on business. The law does not state what would occur if a business refuses or is unable to complete ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liposome
A liposome is a small artificial vesicle, spherical in shape, having at least one lipid bilayer. Due to their hydrophobicity and/or hydrophilicity, biocompatibility, particle size and many other properties, liposomes can be used as drug delivery vehicles for administration of pharmaceutical drugs and nutrients, such as lipid nanoparticles in mRNA vaccines, and DNA vaccines. Liposomes can be prepared by disrupting biological membranes (such as by sonication). Liposomes are most often composed of phospholipids, especially phosphatidylcholine and cholesterol, but may also include other lipids, such as egg, phosphatidylethanolamine, as long as they are compatible with lipid bilayer structure. A liposome design may employ surface ligands for attaching to unhealthy tissue. The major types of liposomes are the multilamellar vesicle (MLV, with several lamellar phase lipid bilayers), the small unilamellar liposome vesicle (SUV, with one lipid bilayer), the large unilamellar vesicle ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Novavax COVID-19 Vaccine
The Novavax COVID-19 vaccine, sold under the brand names Nuvaxovid and Covovax, among others, is a subunit COVID-19 vaccine developed by Novavax and the Coalition for Epidemic Preparedness Innovations (CEPI). Full results from Nuvaxovid's pivotal phase III trial were published in December 2021. Medical uses The Novavax COVID19 vaccine is indicated for active immunization to prevent COVID19 caused by SARS-CoV-2. Efficacy A vaccine is generally considered effective if the estimate is ≥50% with a >30% lower limit of the 95% confidence interval. Efficacy is closely related to effectiveness, which is generally expected to slowly decrease over time. In December 2021, Novavax reported, that its phase III trial showed the vaccine achieved its primary endpoint of preventing infection at least seven days after the second dose. Overall efficacy against different SARS-CoV-2s was 90.4% and efficacy against moderate-to-severe disease was 100%. Trials were conducted before the eme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunologic Adjuvant
In immunology, an adjuvant is a substance that increases or modulates the immune response to a vaccine. The word "adjuvant" comes from the Latin word ''adiuvare'', meaning to help or aid. "An immunologic adjuvant is defined as any substance that acts to accelerate, prolong, or enhance antigen-specific immune responses when used in combination with specific vaccine antigens." In the early days of vaccine manufacture, significant variations in the efficacy of different batches of the same vaccine were correctly assumed to be caused by contamination of the reaction vessels. However, it was soon found that more scrupulous cleaning actually seemed to ''reduce'' the effectiveness of the vaccines, and some contaminants actually enhanced the immune response. There are many known adjuvants in widespread use, including aluminium salts, oils and virosomes. Overview Adjuvants in immunology are often used to modify or augment the effects of a vaccine by stimulating the immune system to re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, Prescription drug, prescription and Over-the-counter drug, over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, Animal feed, animal foods & feed and Veterinary medicine, veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C), but the agency also enforces other laws, notably Section 361 of the Public Health Service Act, as well as associated regulations. Much of this regulatory-enforcement work is not d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
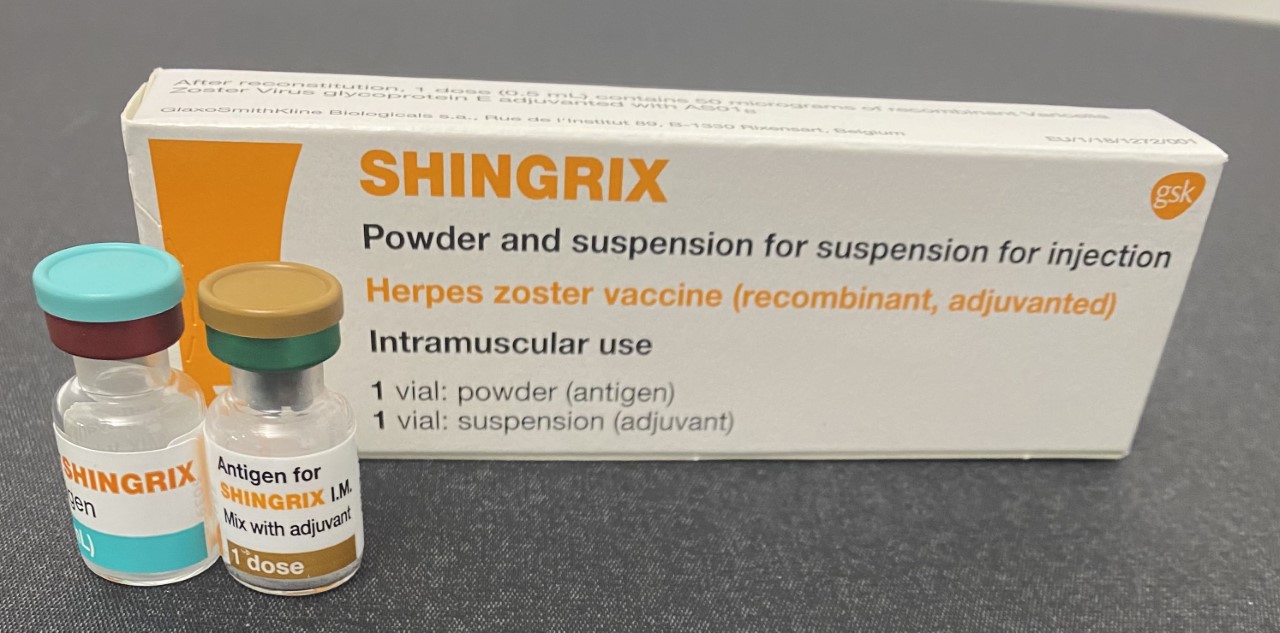
Shingrix
A zoster vaccine is a vaccine that reduces the incidence of herpes zoster (shingles), a disease caused by reactivation of the varicella zoster virus, which is also responsible for chickenpox. Shingles provokes a painful rash with blisters, and can be followed by chronic pain (postherpetic neuralgia), as well as other complications. Older people are more often affected, as are people with weakened immune systems (immunosuppression). Both shingles and postherpetic neuralgia can be prevented by vaccination. Two zoster vaccines have been approved for use in people over 50 years old. Shingrix ( GSK) is a recombinant subunit vaccine which has been used in many countries since 2017. Zostavax (Merck), in use since 2006, is an attenuated vaccine which consists of a larger-than-normal dose of chickenpox vaccine. Unlike Shingrix, Zostavax is not suitable for people with immunosuppression or diseases that affect the immune system. Zostavax was discontinued in the United States in November ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trial
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small pilot studies, and subsequently conduct progressively larger scale comparative studies. Clinical trials can vary i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.jpg)
.jpg)