|
Pudovik Reaction
In organophosphorus chemistry, the Pudovik reaction is a method for preparing α-aminomethylphosphonates. Under basic conditions, the phosphorus–hydrogen bond of a dialkylphosphite, (RO)2P(O)H, adds across the carbon–nitrogen double bond of an imine (a hydrophosphonylation reaction). The reaction is closely related to the three-component Kabachnik–Fields reaction, where an amine, phosphite, and an organic carbonyl compound are condensed, which was reported independently by Martin Kabachnik and Ellis Fields in 1952. In the Pudovik reaction, a generic imine, RCH=NR', would react with a phosphorous reagent like diethylphosphite as follows: :RCH=NR' + (EtO)2P(O)H → (EtO)2P(O)CHR-NHR' In addition to the Lewis-acid catalyzed Pudovik reaction, the reaction may be carried out in the presence of chiral amine bases. Catalytic amounts of quinine Quinine is a medication used to treat malaria and babesiosis. This includes the treatment of malaria due to ''Plasmod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphorus Chemistry
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX (nerve agent), VX nerve agents. Organophosphorus chemistry is the corresponding science of the properties and reactivity of organophosphorus compounds. Phosphorus, like nitrogen, is in pnictogen, group 15 of the periodic table, and thus phosphorus compounds and nitrogen compounds have many similar properties. The definition of organophosphorus compounds is variable, which can lead to confusion. In industrial and environmental chemistry, an organophosphorus compound need contain only an organic substituent, but need not have a direct phosphorus-carbon (P-C) bond. Thus a large proportion of pesticides (e.g., malathion), are often included in this class of compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aminophosphonate
Aminophosphonates are organophosphorus compounds with the formula (RO)2P(O)CR'2NR"2. These compounds are structural analogues of amino acids in which a carboxylic moiety is replaced by phosphonic acid or related groups. Acting as antagonists of amino acids, they inhibit enzymes involved in amino acid metabolism and thus affect the physiological activity of the cell. These effects may be exerted as antibacterial, plant growth regulatory or neuromodulatory. They can act as ligands, and heavy metal complexes with aminophosphonates have had medical applications investigated. Phosphonates are more difficult to hydrolyse than phosphates. Preparation Aminophosphonates are often prepared by hydrophosphonylation, usually the condensation of imines and phosphorous acid. In the Pudovik reaction or Kabachnik–Fields reaction the esters of phosphorous acid are employed, e.g. diphenylphosphite. Because these compounds are of pharmaceutical interest, methods have been developed to induce the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
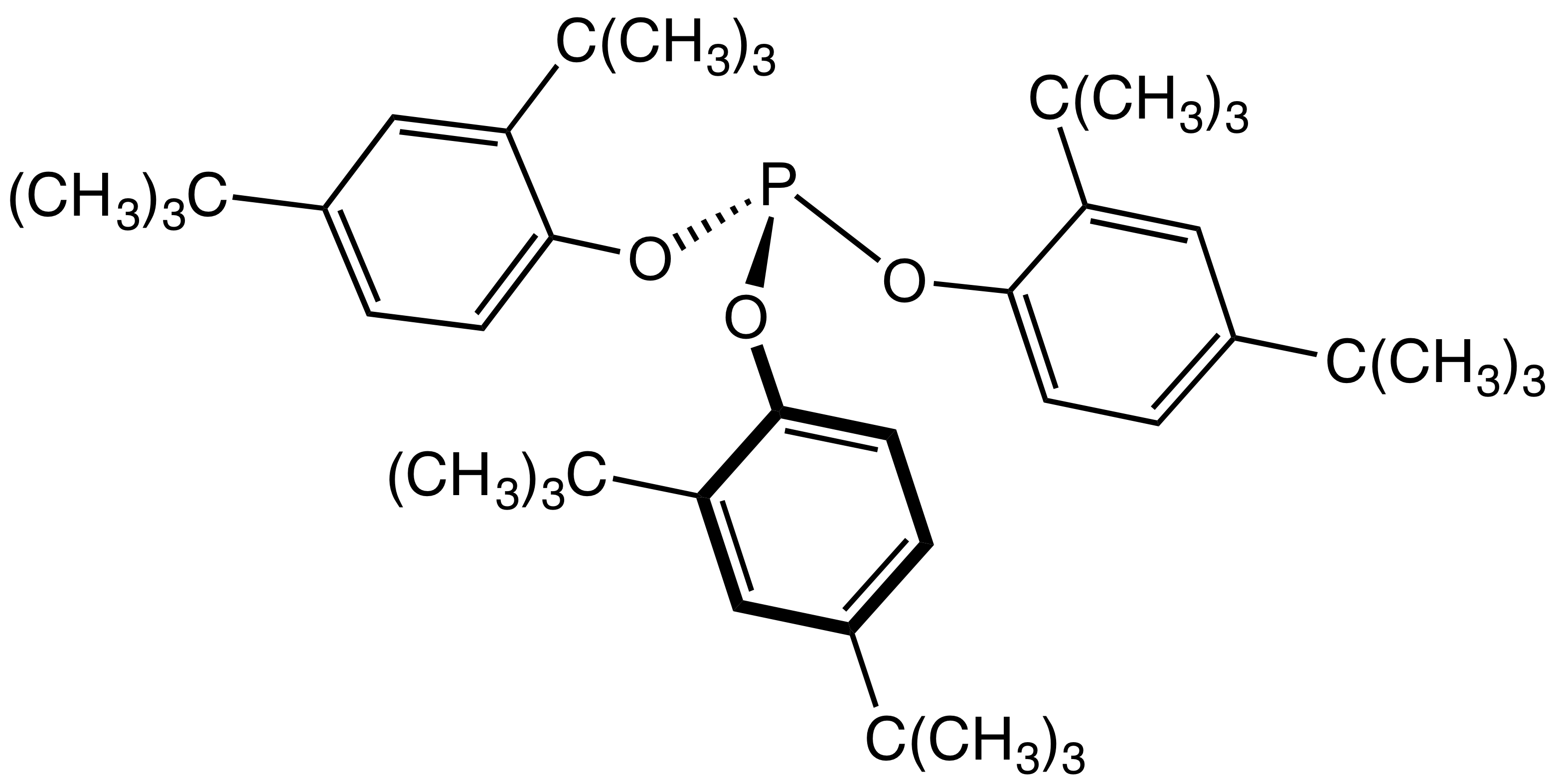
Phosphite Ester
The general structure of a phosphite ester showing the lone pairs on the P In organic chemistry, a phosphite ester or organophosphite usually refers to an organophosphorous compound with the formula P(OR)3. They can be considered as esters of an unobserved tautomer phosphorous acid, H3PO3, with the simplest example being trimethylphosphite, P(OCH3)3. Some phosphites can be considered esters of the dominant tautomer of phosphorous acid (HP(O)(OH)2). The simplest representative is dimethylphosphite with the formula HP(O)(OCH3)2. Both classes of phosphites are usually colorless liquids. Synthesis ;From PCl3 Phosphite esters are typically prepared by treating phosphorus trichloride with an alcohol. Depending on the synthetic details, this alcoholysis can give the diorganophosphites: :PCl3 + 3 C2H5OH → (C2H5O)2P(O)H + 2 HCl + C2H5Cl Alternatively, when the alcoholysis is conducted in the presence of proton acceptors, one obtains the C3-symmetric trialkoxy derivatives: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Addition Reaction
In organic chemistry, an addition reaction is, in simplest terms, an organic reaction where two or more molecules combine to form a larger one (the adduct).. Addition reactions are limited to chemical compounds that have multiple bonds, such as molecules with carbon–carbon double bonds (alkenes), or with triple bonds (alkynes), and compounds that have rings, which are also considered points of unsaturation. Molecules containing carbon— hetero double bonds like carbonyl () groups, or imine () groups, can undergo addition, as they too have double-bond character. An addition reaction is the reverse of an elimination reaction. For instance, the hydration of an alkene to an alcohol is reversed by dehydration. There are two main types of polar addition reactions: electrophilic addition and nucleophilic addition. Two non-polar addition reactions exist as well, called free-radical addition and cycloadditions. Addition reactions are also encountered in polymerizations and called a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions. Structure For ketimines and aldimines, respectively, the five core atoms (C2C=NX and C(H)C=NX, X = H or C) are coplanar. Planarity results from the sp2-hybridization of the mutually double-bonded carbon and the nitrogen atoms. The C=N distance is 1.29-1.31 Å for nonconjugated imines and 1.35 Å for conjugated imines. By contrast, C-N distances in amines and nitriles are 1.47 and 1.16 Å, respectively. Rotation about the C=N bond is slow. Using NMR spectroscopy, both E- and Z-isomers of aldimines have been detected. Owing to steric effects, the E isomer is favored. Nomenclature and classification The term "imine" was coine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrophosphonylation
In chemistry hydrophosphonylation refers to any reaction where addition across a double bond generates a phosphonate (RP(O)(OR')2) group. Examples include the Kabachnik–Fields reaction, where a dialkylphosphite reacts across an imine to form an aminophosphonate. The reaction is catalyzed by bases and is subject to organocatalysis. Important compounds generated by this reaction include the common herbicide glyphosate. : Hydrophosphonylation reactions *Kabachnik–Fields reaction * Pudovik reaction * Abramov reaction See also * Hydrophosphination Hydrophosphination is the insertion of a double bond, carbon-carbon multiple bond into a phosphorus-hydrogen bond forming a new phosphorus-carbon bond. Like other hydrofunctionalizations, the rate and regiochemistry of the insertion reaction is in ... - the addition of a phosphine derivative (PHR2) across a double bond References {{Reflist Addition reactions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Reactions
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis, organic reactions are used in the construction of new organic molecules. The production of many man-made chemicals such as drugs, plastics, food additives, fabrics depend on organic reactions. The oldest organic reactions are combustion of organic fuels and saponification of fats to make soap. Modern organic chemistry starts with the Wöhler synthesis in 1828. In the history of the Nobel Prize in Chemistry awards have been given for the invention of specific organic reactions such as the Grignard reaction in 1912, the Diels-Alder reaction in 1950, the Wittig reaction in 1979 and olefin metathesis in 2005. Classifications Organic chemistry has a strong tradition of naming a specific reac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kabachnik–Fields Reaction
In organophosphorus chemistry, the Kabachnik–Fields reaction is a three-component organic reaction forming aminophosphonate, α-aminomethylphosphonates from an amine, a carbonyl compound, and a phosphite ester, dialkyl phosphonate, (RO)2P(O)H (that are also called dialkylphosphites). Aminophosphonates are synthetic targets of some importance as phosphorus analog (chemistry), analogues of α-amino acids (a bioisosterism, bioisostere). This multicomponent reaction was independently discovered by and Ellis K. Fields in 1952. The reaction is very similar to the two-component Pudovik reaction, which involves condensation of the phosphite and a preformed imine. : The first step in this reaction is the formation of an imine, followed by a hydrophosphonylation step where the phosphonate P-H bond across the C=N double bond. The starting carbonyl component is usually an aldehyde and sometimes a ketone. The reaction can be accelerated with a combination of dehydrating reagent and Lewis acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl Compound
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound. The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl). The remainder of this article concerns itself with the organic chemistry definition of carbonyl, where carbon and oxygen share a double bond. Carbonyl compounds In organic chemistry, a carbonyl group characterizes the following types of compounds: Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, hydroxamates, and isocyanates. Examples of inorganic carbonyl compounds are carbon dioxide and carbonyl sulfide. A sp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecules (journal)
''Molecules'' is a peer-reviewed open access scientific journal that focuses on all aspects of chemistry and materials science. It was established in March 1996 and is published monthly by MDPI. From 1997 to 2001, ''Molbank'' was published as a section of the journal, before splitting into its own journal. The editor-in-chief is Farid Chemat. ''Molecules'' was initially published by Springer-Verlag. In December 1996, Shu-Kun Lin resigned as editor and relaunched the journal with Molecular Diversity Preservation International (MDPI). Springer initially sued over naming rights, but eventually dropped the suit. Abstracting and indexing The journal is abstracted and indexed in: According to the ''Journal Citation Reports'', the journal has a 2021 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Doklady Akademii Nauk SSSR
The ''Proceedings of the USSR Academy of Sciences'' (russian: Доклады Академии Наук СССР, ''Doklady Akademii Nauk SSSR'' (''DAN SSSR''), french: Comptes Rendus de l'Académie des Sciences de l'URSS) was a Soviet journal that was dedicated to publishing original, academic research papers in physics, mathematics, chemistry, geology, and biology. It was first published in 1933 and ended in 1992 with volume 322, issue 3. Today, it is continued by ''Doklady Akademii Nauk'' (russian: Доклады Академии Наук), which began publication in 1992. The journal is also known as the ''Proceedings of the Russian Academy of Sciences (RAS)''. ''Doklady'' has had a complicated publication and translation history. A number of translation journals exist which publish selected articles from the original by subject section; these are listed below. History The Russian Academy of Sciences dates from 1724, with a continuous series of variously named publications dat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |