|
Pregnane X Receptor Antagonists
Pregnane, also known as 17β-ethylandrostane or as 10β,13β-dimethyl-17β-ethylgonane, is a C21 steroid and, indirectly, a parent of progesterone. It is a parent hydrocarbon for two series of steroids stemming from 5α-pregnane (originally allopregnane) and 5β-pregnane (17β-ethyletiocholane). It has a gonane core. 5β-Pregnane is the parent of pregnanediones, pregnanolones, and pregnanediols, and is found largely in urine as a metabolic product of 5β-pregnane compounds. Pregnanes Pregnanes are steroid derivatives with carbons present at positions 1 through 21. Most biologically significant pregnane derivatives fall into one of two groups: pregnenes and pregnadienes. Another class is pregnatrienes. Pregnenes Pregnenes have a double bond. Examples include: * Cortisone * Hydrocortisone * Progesterone Pregnadienes Pregnadienes have two double bonds. Examples include: * Cyproterone acetate * Danazol * Fluocinonide See also * 5β-Pregnane * Pregnanedione * Pregnanedio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Union Of Pure And Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. This administrative office is headed by IUPAC's executive director, currently Lynn Soby. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steroid Numbering
A steroid is a biologically active organic compound with four rings arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as signaling molecules. Hundreds of steroids are found in plants, animals and fungi. All steroids are manufactured in cells from the sterols lanosterol (opisthokonts) or cycloartenol (plants). Lanosterol and cycloartenol are derived from the cyclization of the triterpene squalene. The steroid core structure is typically composed of seventeen carbon atoms, bonded in four " fused" rings: three six-member cyclohexane rings (rings A, B and C in the first illustration) and one five-member cyclopentane ring (the D ring). Steroids vary by the functional groups attached to this four-ring core and by the oxidation state of the rings. Sterols are forms of steroids with a hydroxy group at position three and a skeleton derived from cholestane. ''Also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
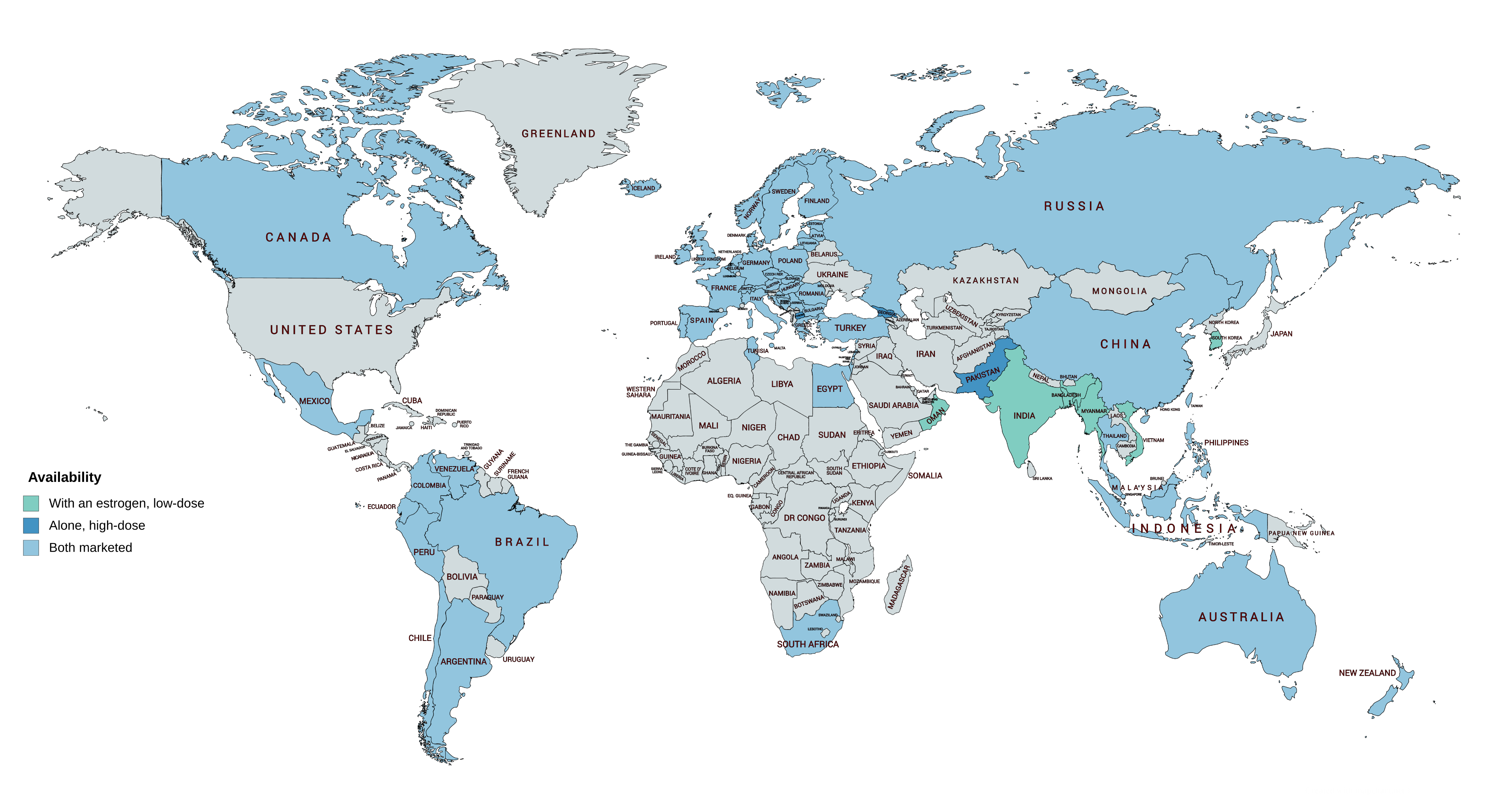
Fluocinonide
Fluocinonide (sold under various brand names) is a potent glucocorticoid used topically as an anti-inflammatory agent for the treatment of skin disorders such as eczema and seborrhoeic dermatitis. It relieves itching, redness, dryness, crusting, scaling, inflammation, and discomfort. A common potential adverse effect is skin atrophy (thinning of the skin). Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal axis (HPA) suppression, manifestations of Cushing's syndrome, hyperglycemia, and glucosuria Glycosuria is the excretion of glucose into the urine. Ordinarily, urine contains no glucose because the kidneys are able to reabsorb all of the filtered glucose from the tubular fluid back into the bloodstream. Glycosuria is nearly always caused .... In 2020, it was the 280th most commonly prescribed medication in the United States, with more than 1million prescriptions. Veterinary uses Fluocinonide is used in veterinary medic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Danazol
Danazol, sold as Danocrine and other brand names, is a medication used in the treatment of endometriosis, fibrocystic breast disease, hereditary angioedema and other conditions. It is taken by mouth. The use of danazol is limited by masculinizing side effects such as acne, excessive hair growth, and voice deepening. Danazol has a complex mechanism of action, and is characterized as a weak androgen and anabolic steroid, a weak progestogen, a weak antigonadotropin, a weak steroidogenesis inhibitor, and a functional antiestrogen. Danazol was discovered in 1963 and was introduced for medical use in 1971. Due to their improved side-effect profiles, particularly their lack of masculinizing side effects, danazol has largely been replaced by gonadotropin-releasing hormone analogues (GnRH analogues) in the treatment of endometriosis. Medical uses Danazol is used primarily in the treatment of endometriosis. It has also been used – mostly off-label – for other indications, name ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyproterone Acetate
Cyproterone acetate (CPA), sold alone under the brand name Androcur or Ethinylestradiol/cyproterone acetate, with ethinylestradiol under the brand names Diane or Diane-35 among others, is an antiandrogen and progestin medication used in the treatment of androgen-dependent conditions such as acne, hirsutism, excessive body hair growth, precocious puberty, early puberty, and prostate cancer, as a component of feminizing hormone therapy for transgender women, and in oral contraceptive, birth control pills. It is formulated and used both alone and in combination with an estrogen (medication), estrogen. CPA is taken Oral administration, by mouth one to three times per day. Common side effects of high-dose CPA in men include gynecomastia (breast development) and feminization (biology), feminization. In both men and women, possible side effects of CPA include hypogonadism, low sex hormone levels, reversible infertility, sexual dysfunction, fatigue (medical), fatigue, depression (mood), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyproterone Acetate
Cyproterone acetate (CPA), sold alone under the brand name Androcur or Ethinylestradiol/cyproterone acetate, with ethinylestradiol under the brand names Diane or Diane-35 among others, is an antiandrogen and progestin medication used in the treatment of androgen-dependent conditions such as acne, hirsutism, excessive body hair growth, precocious puberty, early puberty, and prostate cancer, as a component of feminizing hormone therapy for transgender women, and in oral contraceptive, birth control pills. It is formulated and used both alone and in combination with an estrogen (medication), estrogen. CPA is taken Oral administration, by mouth one to three times per day. Common side effects of high-dose CPA in men include gynecomastia (breast development) and feminization (biology), feminization. In both men and women, possible side effects of CPA include hypogonadism, low sex hormone levels, reversible infertility, sexual dysfunction, fatigue (medical), fatigue, depression (mood), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Progesterone
Progesterone (P4) is an endogenous steroid and progestogen sex hormone involved in the menstrual cycle, pregnancy, and embryogenesis of humans and other species. It belongs to a group of steroid hormones called the progestogens and is the major progestogen in the body. Progesterone has a variety of important functions in the body. It is also a crucial metabolic intermediate in the production of other endogenous steroids, including the sex hormones and the corticosteroids, and plays an important role in brain function as a neurosteroid. In addition to its role as a natural hormone, progesterone is also used as a medication, such as in combination with estrogen for contraception, to reduce the risk of uterine or cervical cancer, in hormone replacement therapy, and in feminizing hormone therapy. It was first prescribed in 1934. Biological activity Progesterone is the most important progestogen in the body. As a potent agonist of the nuclear progesterone receptor (nPR) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocortisone
Hydrocortisone is the name for the hormone cortisol when supplied as a medication. Uses include conditions such as adrenocortical insufficiency, adrenogenital syndrome, high blood calcium, thyroiditis, rheumatoid arthritis, dermatitis, asthma, and COPD. It is the treatment of choice for adrenocortical insufficiency. It can be given by mouth, topically, or by injection. Stopping treatment after long-term use should be done slowly. Side effects may include mood changes, increased risk of infection, and edema (swelling). With long-term use common side effects include osteoporosis, upset stomach, physical weakness, easy bruising, and candidiasis (yeast infections). While used, it is unclear if it is safe during pregnancy. Hydrocortisone is a glucocorticoid and works as an anti-inflammatory and by immune suppression. Hydrocortisone was patented in 1936 and approved for medical use in 1941. It is on the World Health Organization's List of Essential Medicines. It is available as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cortisone
Cortisone is a pregnene (21-carbon) steroid hormone. It is a naturally-occurring corticosteroid metabolite that is also used as a pharmaceutical prodrug; it is not synthesized in the adrenal glands. Cortisol is converted by the action of the enzyme corticosteroid 11-beta-dehydrogenase isozyme 2 into the inactive metabolite cortisone, particularly in the kidneys. Cortisone is converted back to the active steroid cortisol by the action of the enzyme 11β-Hydroxysteroid dehydrogenase type 1, particularly in the liver. The term "cortisone" is frequently misused to mean either any corticosteroid or hydrocortisone, which is actually another name for cortisol. Many who speak of receiving a "cortisone shot" or taking "cortisone" are actually receiving hydrocortisone or one of many other, much more potent synthetic corticosteroids; it is unlikely that the drug administered is actually cortisone. Cortisone can be administered as a prodrug, meaning it has to be converted by the body (speci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist between two different elements: for example, in a carbonyl group between a carbon atom and an oxygen atom. Other common double bonds are found in azo compounds (N=N), imines (C=N), and sulfoxides (S=O). In a skeletal formula, a double bond is drawn as two parallel lines (=) between the two connected atoms; typographically, the equals sign is used for this. Double bonds were first introduced in chemical notation by Russian chemist Alexander Butlerov. Double bonds involving carbon are stronger and shorter than single bonds. The bond order is two. Double bonds are also electron-rich, which makes them potentially more reactive in the presence of a strong electron acceptor (as in addition reactions of the halogens). File:Ethene structural.svg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cortisone Structure
Cortisone is a pregnene (21-carbon) steroid hormone. It is a naturally-occurring corticosteroid metabolite that is also used as a pharmaceutical prodrug; it is not synthesized in the adrenal glands. Cortisol is converted by the action of the enzyme corticosteroid 11-beta-dehydrogenase isozyme 2 into the inactive metabolite cortisone, particularly in the kidneys. Cortisone is converted back to the active steroid cortisol by the action of the enzyme 11β-Hydroxysteroid dehydrogenase type 1, particularly in the liver. The term "cortisone" is frequently misused to mean either any corticosteroid or hydrocortisone, which is actually another name for cortisol. Many who speak of receiving a "cortisone shot" or taking "cortisone" are actually receiving hydrocortisone or one of many other, much more potent synthetic corticosteroids; it is unlikely that the drug administered is actually cortisone. Cortisone can be administered as a prodrug, meaning it has to be converted by the body ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |