|
Potassium Tetraphenylborate
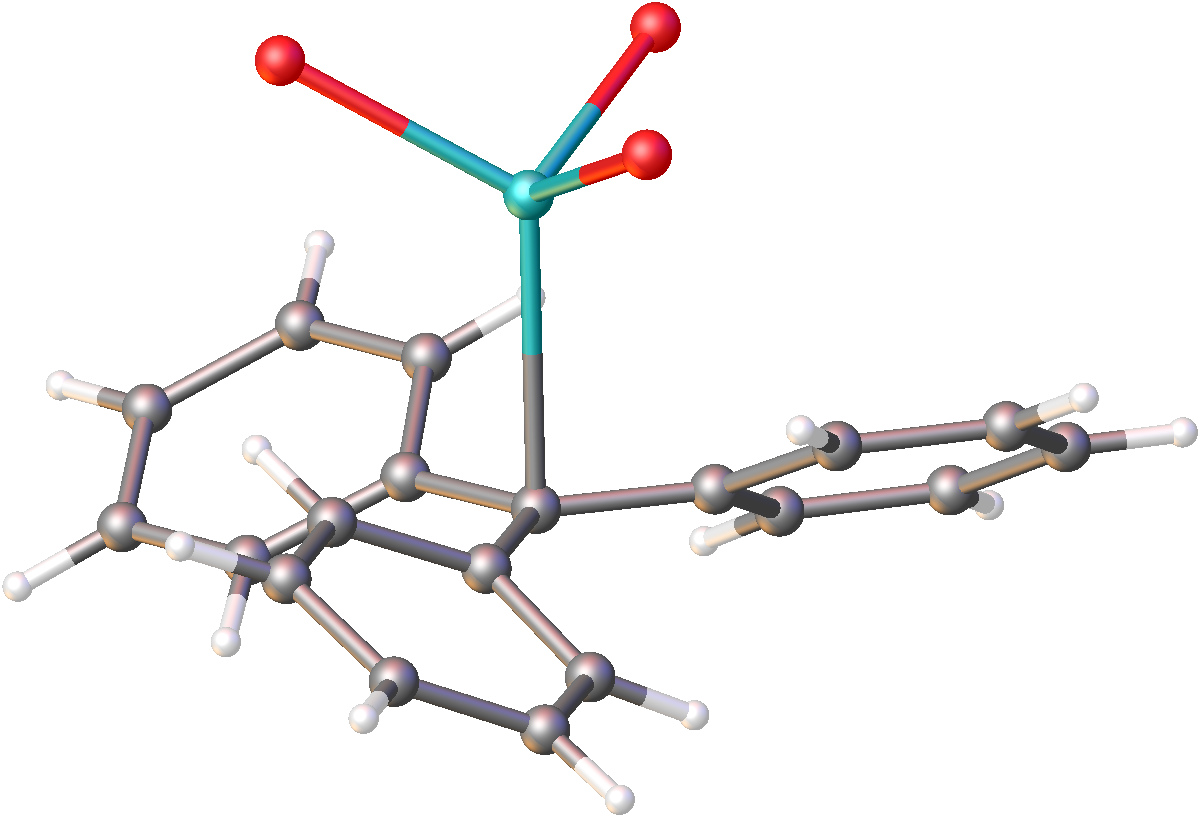
Potassium tetraphenylborate is the salt with the formula KB(C6H5)4). It is a colourless salt that is a rare example of a water-insoluble salt of potassium. The salt has a low solubility in water of only 1.8×10−4 g/L. It is, however, soluble in organic solvents. The insolubility of this compound has been used to determine the concentration of potassium ions by precipitation and gravimetric analysis: : K+ + NaB(Ph)4 → KB(Ph)4 + Na+ The compound adopts a polymeric structure with bonds between the phenyl rings and potassium. As such it is classified as an organopotassium compound Organosodium chemistry is the chemistry of organometallic compounds containing a carbon to sodium chemical bond. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compounds, which are ....Ulrich Behrens, Frank Hoffmann, and Falk Olbrich "Solid-State Structures of Base-Free Lithium and Sodium Tetraphenylborates at Room and Low T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, that is easily removed to create an ion with a positive charge – a cation, that combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in sea water (which is 0.04% potassium by weight), and occurs in many minerals such as orthoclase, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell. The quantity of solute that can dissolve in a specific volume of solvent varies with temperature. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners (toluene, turpentine); as nail polish removers and solvents of glue (acetone, methyl acetate, ethyl acetate); in spot removers (hexane, petrol ether); in detergents ( citrus terpenes); and in perfumes (ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas industries, including in chemical synt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Precipitation (chemistry)
In an aqueous solution, precipitation is the process of transforming a dissolved chemical substance, substance into an insoluble solid from a Supersaturated solution, super-saturated solution. The solid formed is called the precipitate. In case of an inorganic chemical reaction leading to precipitation, the chemical reagent causing the solid to form is called the ''precipitant''. The clear liquid remaining above the precipitated or the centrifuged solid phase is also called the 'supernate' or 'supernatant'. The notion of precipitation can also be extended to other domains of chemistry (organic chemistry and biochemistry) and even be applied to the solid phases (''e.g.'', metallurgy and alloys) when solid impurities Segregation (materials science), segregate from a solid phase. Supersaturation The precipitation of a compound may occur when its concentration exceeds its solubility. This can be due to temperature changes, solvent evaporation, or by mixing solvents. Precipitatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gravimetric Analysis
Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte (the ion being analyzed) based on its mass. The principle of this type of analysis is that once an ion's mass has been determined as a unique compound, that known measurement can then be used to determine the same analyte's mass in a mixture, as long as the relative quantities of the other constituents are known. The four main types of this method of analysis are ''precipitation'', ''volatilization'', ''electro-analytical'' and ''miscellaneous physical method''. The methods involve changing the phase of the analyte to separate it in its pure form from the original mixture and are quantitative measurements. Precipitation method The precipitation method is the one used for the determination of the amount of calcium in water. Using this method, an excess of oxalic acid, H2C2O4, is added to a measured, known volume of water. By adding a reagent, here ammoni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anal
Anal may refer to: Related to the anus *Related to the anus of animals: ** Anal fin, in fish anatomy ** Anal vein, in insect anatomy ** Anal scale, in reptile anatomy *Related to the human anus: ** Anal sex, a type of sexual activity involving stimulation of the anus ** Anal stage, a term used by Sigmund Freud to describe the development during the second year of life ** Anal expulsive, people who have a carefree attitude ** Anal retentive, a person overly uptight or distressed over ordinarily minor problems Places * Anal Island, an island of the Marshall Islands * Añal, New Mexico, a ghost town Other uses * Anāl people, an ethnic group of northeast India and Myanmar **Anāl language, the Sino-Tibetan language they speak * Ammonal, or ANAL, an explosive made from ammonium nitrate (AN) and aluminium (AL) powder * ''All Nippon Air Line'', a 2008 boys love manga * Anal Arasu, Indian fight master/action choreographer See also * IANAL, a colloquial acronym for "I am not a law ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organopotassium Compound
Organosodium chemistry is the chemistry of organometallic compounds containing a carbon to sodium chemical bond. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compounds, which are commercially available and exhibit more convenient reactivity. The principal organosodium compound of commercial importance is sodium cyclopentadienide. Sodium tetraphenylborate can also be classified as an organosodium compound since in the solid state sodium is bound to the aryl groups. Organometal bonds in group 1 are characterised by high polarity with corresponding high nucleophilicity on carbon. This polarity results from the disparate electronegativity of carbon (2.55) and that of lithium 0.98, sodium 0.93 potassium 0.82 rubidium 0.82 caesium 0.79). The carbanionic nature of organosodium compounds can be minimized by resonance stabilization, for example, Ph3CNa. One consequence of the highly polarized Na-C bond is that simpl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Compounds
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, that is easily removed to create an ion with a positive charge – a cation, that combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in sea water (which is 0.04% potassium by weight), and occurs in many minerals such as orthoclase, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |