|
Potassium Hydride
Potassium hydride, KH, is the inorganic compound of potassium and hydrogen. It is an alkali metal hydride. It is a white solid, although commercial samples appear gray. It is a powerful superbase that is useful in organic synthesis. It is sold commercially as a slurry (~35%) in mineral oil or sometimes paraffin wax to facilitate dispensing. Preparation Potassium hydride is produced by direct combination of the metal and hydrogen: : This reaction was discovered by Humphry Davy soon after his 1807 discovery of potassium, when he noted that the metal would vaporize in a current of hydrogen when heated just below its boiling point.Humphry Davy (1808), ''The Bakerian Lecture on some new phenomena of chemical changes produced by electricity, particularly the decomposition of fixed alkalies, and the exhibition of the new substances which constitute their bases; and on the general nature of alkaline bodies.'' Philosophical Transactions of the Royal Society, volume 88, pages 1–44. In ''Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a precursor to the manufacture of chemicals with more complex structure, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity. History Discovery The word "''benzene''" derives from "''gum benzoin''" (benzoin res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
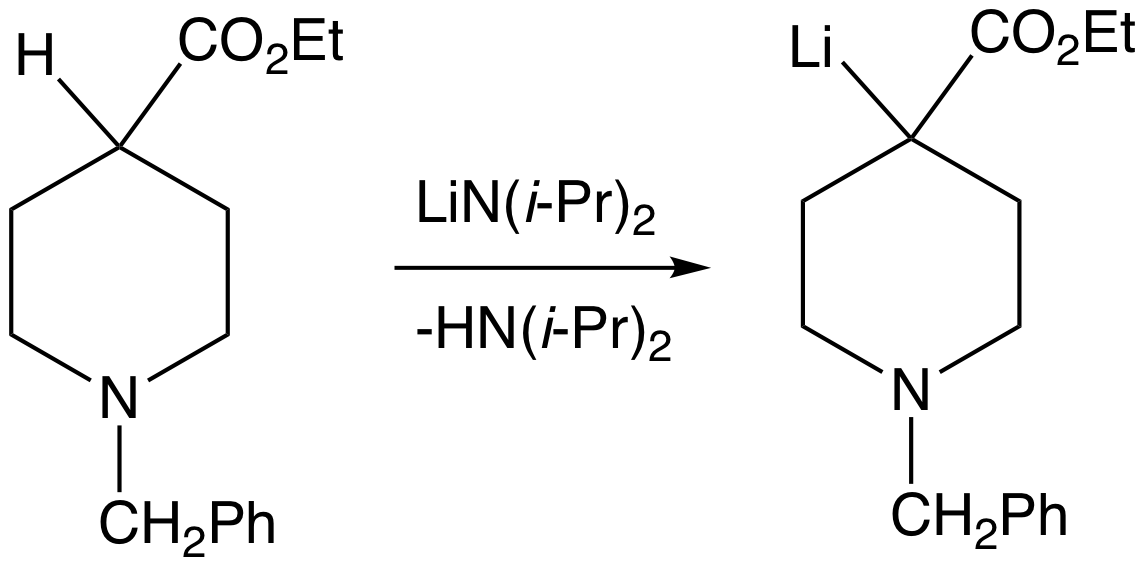
Superbase
A superbase is a compound that has a particularly high affinity for protons. Superbases are of theoretical interest and potentially valuable in organic synthesis. Superbases have been described and used since the 1850s.''Superbases for Organic Synthesis'' Ed. Ishikawa, T., John Wiley and Sons, Ltd.: West Sussex, UK. 2009. Definitions Generically IUPAC defines a superbase as a "compound having a very high basicity, such as lithium diisopropylamide." Superbases are often defined in two broad categories, organic and organometallic. Organic superbases are charge-neutral compounds with basicities greater than that of proton sponge (pKBH+ = 18.6 in MeCN)." In a related definition: any species with a higher absolute proton affinity (APA = 245.3 kcal/mol) and intrinsic gas phase basicity (GB = 239 kcal/mol) than proton sponge. Common superbases of this variety feature amidine, guanidine, and phosphazene functional groups. Strong superbases can be designed by utilizing multiple intram ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Compounds
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, that is easily removed to create an ion with a positive charge – a cation, that combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in sea water (which is 0.04% potassium by weight), and occurs in many minerals such as orthoclase, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Hydrides
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed. Almost all of the elements form binary compounds with hydrogen, the exceptions being He, Ne, Ar, Kr, Pm, Os, Ir, Rn, Fr, and Ra. Exotic molecules such as positronium hydride have also been made. Bonds Bonds between hydrogen and the other elements range from highly to somewhat covalent. Some hydrides, e.g. boron hydrides, do not conform to classical electron-counting rules and the bonding is described in terms of multi-centered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enolates
In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl () compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds. Bonding and structure Enolate anions are electronically related to allyl anions. The anionic charge is delocalized over the oxygen and the two carbon sites. Thus they have the character of both an alkoxide and a carbanion. Although they are often drawn as being simple salts, in fact they adopt complicated structures often featuring aggregates. Preparation Deprotonation of enolizable ketones, aromatic alcohols, aldehydes, and esters gives enolates. With strong bases, the deprotonation is quantitative. Typically enolates are generated from using lithium diisopropylamide (LDA). Often, as in conventional Claisen condensations, Mannich reactions, and aldol condensations, enolates are generated in low concentrations with alkoxide bases. Under such conditions, they exist in low concent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.edu/hjakubowski/classes/ch331/protstructure/PS_2A3_AA_Charges.html, accessed 12/2/2020 The species formed is the conjugate base of that acid. The complementary process, when a proton is added (transferred) to a Brønsted–Lowry base, is protonation (or hydronation). The species formed is the conjugate acid of that base. A species that can either accept or donate a proton is referred to as amphiprotic. An example is the H2O (water) molecule, which can gain a proton to form the hydronium ion, H3O+, or lose a proton, leaving the hydroxide ion, OH−. The relative ability of a molecule to give up a proton is measured by its p''K''a value. A low p''K''a value indicates that the compound is acidic and will easily give up its proton to a base ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydride
Sodium hydride is the chemical compound with the empirical formula Na H. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in contrast to molecular hydrides such as borane, methane, ammonia, and water. It is an ionic material that is insoluble in organic solvents (although soluble in molten Na), consistent with the fact that H− ions do not exist in solution. Because of the insolubility of NaH, all reactions involving NaH occur at the surface of the solid. Basic properties and structure NaH is produced by the direct reaction of hydrogen and liquid sodium.Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. . Pure NaH is colorless, although samples generally appear grey. NaH is ca. 40% denser than Na (0.968 g/cm3). NaH, like LiH, KH, RbH, and CsH, adopts the NaCl crystal structure. In this motif, each Na+ ion is surrounded by six H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions . Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burns. It is highly soluble in water, and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates . The monohydrate crystallizes from water solutions between 12.3 and 61.8 °C. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, sodium hydroxide is frequently used alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students. Sodium hydroxide is used in many industries: in the manufacture of pulp and paper, textiles, drinking water, soaps and deterge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Humphry Davy
Sir Humphry Davy, 1st Baronet, (17 December 177829 May 1829) was a British chemist and inventor who invented the Davy lamp and a very early form of arc lamp. He is also remembered for isolating, by using electricity, several elements for the first time: potassium and sodium in 1807 and calcium, strontium, barium, magnesium and boron the following year, as well as for discovering the elemental nature of chlorine and iodine. Davy also studied the forces involved in these separations, inventing the new field of electrochemistry. Davy is also credited to have been the first to discover clathrate hydrates in his lab. In 1799 he experimented with nitrous oxide and was astonished at how it made him laugh, so he nicknamed it "laughing gas" and wrote about its potential anaesthetic properties in relieving pain during surgery. Davy was a baronet, President of the Royal Society (PRS), Member of the Royal Irish Academy (MRIA), Fellow of the Geological Society (FGS), and a member ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paraffin Wax
Paraffin wax (or petroleum wax) is a soft colorless solid derived from petroleum, coal, or oil shale that consists of a mixture of hydrocarbon molecules containing between 20 and 40 carbon atoms. It is solid at room temperature and begins to melt above approximately , and its boiling point is above . Common applications for paraffin wax include lubrication, electrical insulation, and candles; dyed paraffin wax can be made into crayons. It is distinct from kerosene and other petroleum products that are sometimes called paraffin. Un-dyed, unscented paraffin candles are odorless and bluish-white. Paraffin wax was first created by Carl Reichenbach in Germany in 1830 and marked a major advancement in candlemaking technology, as it burned more cleanly and reliably than tallow candles and was cheaper to produce. In chemistry, ''paraffin'' is used synonymously with ''alkane'', indicating hydrocarbons with the general formula C''n''H2''n''+2. The name is derived from Latin ''parum'' (" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mineral Oil
Mineral oil is any of various colorless, odorless, light mixtures of higher alkanes from a mineral source, particularly a distillate of petroleum, as distinct from usually edible vegetable oils. The name 'mineral oil' by itself is imprecise, having been used for many specific oils over the past few centuries. Other names, similarly imprecise, include 'white oil', 'paraffin oil', ' liquid paraffin' (a highly refined medical grade), (Latin), and 'liquid petroleum'. Most often, mineral oil is a liquid by-product of refining crude oil to make gasoline and other petroleum products. This type of mineral oil is a transparent, colorless oil, composed mainly of alkanes and cycloalkanes, related to petroleum jelly. It has a density of around . Nomenclature Some of the imprecision in the definition of the names used for mineral oil (such as 'white oil') reflects usage by consumers and merchants who did not know, and usually had no need of knowing, the oil's precise chemical makeup ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: ''total synthesis'', ''semisynthesis'', and ''methodology''. Total synthesis A total synthesis is the complete chemical synthesis of complex organic molecules from simple, commercially available petrochemical or natural precursors. Total synthesis may be accomplished either via a linear or convergent approach. In a ''linear'' synthesis—often adequate for simple structures—several steps are performed one after another until the molecule is complete; the chemical compounds made in each step are called synthetic intermediates. Most often, each step in a synthesis refers to a separate rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2.png)