|
Polymerization Induced Phase Separation
Polymerization-induced phase separation (PIPS) is the occurrence of phase separation in a multicomponent mixture induced by the polymerization of one or more components. The increase in molecular weight of the reactive component renders one or more components to be mutually immiscible in one another, resulting in spontaneous phase segregation. Types Polymerization-induced phase separation can be initiated either through thermally induced polymerization or photopolymerization. The process general occurs through spinodal decomposition Spinodal decomposition is a mechanism by which a single thermodynamic phase spontaneously separates into two phases (without nucleation). Decomposition occurs when there is no thermodynamic barrier to phase separation. As a result, phase separatio ..., commonly resulting in the formation of co-continuous phases. Control over morphology The morphology of the final phase separated structures are generally random owing to the stochastic nature of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Separation
Phase separation is the creation of two distinct phases from a single homogeneous mixture. The most common type of phase separation is between two immiscible liquids, such as oil and water. Colloids are formed by phase separation, though not all phase separations forms colloids - for example oil and water can form separated layers under gravity rather than remaining as microscopic droplets in suspension. Phase separation in cold gases A mixture of two helium isotopes (helium-3 and helium-4) in a certain range of temperatures and concentrations separates into parts. The initial mix of the two isotopes spontaneously separates into ^He-rich and ^3He-rich regions. Phase separation also exists in ultracold gas systems. It has been shown experimentally in a two-component ultracold Fermi gas case. The phase separation can compete with other phenomena as vortex lattice formation or an exotic Fulde-Ferrell-Larkin-Ovchinnikov phase. See also * Biomolecular condensate * Collo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer, monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many forms of polymerization and different systems exist to categorize them. In chemical compounds, polymerization can occur via a variety of reaction mechanisms that vary in complexity due to the functional groups present in the reactants and their inherent steric effects. In more straightforward polymerizations, alkenes form polymers through relatively simple free-radical reaction, radical reactions; in contrast, reactions involving substitution at a carbonyl group require more complex synthesis due to the way in which reactants polymerize. Alkanes can also be polymerized, but only with the help of strong acids. As alkenes can polymerize in somewhat straightforward radical reactions, they form useful compounds such as polyethylene and p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immiscible
Miscibility () is the property of two chemical substance, substances to mix in all mixing ratio, proportions (that is, to fully dissolution (chemistry), dissolve in each other at any concentration), forming a homogeneity and heterogeneity, homogeneous mixture (a Solution (chemistry), solution). The term is most often applied to liquids but also applies to solids and gases. For example, water and ethanol are miscible because they mix in all proportions. By contrast, substances are said to be immiscible if there are certain proportions in which the mixture does not form a solution. For one example, oil is not soluble in water, so these two solvents are immiscible. As another example, butanone (methyl ethyl ketone) is significantly soluble in water, but these two solvents are also immiscible because in some proportions the mixture will separate into two Phase (matter), phases. Organic compounds In organic compounds, the Concentration#Mass percentage (fraction), weight percent of h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
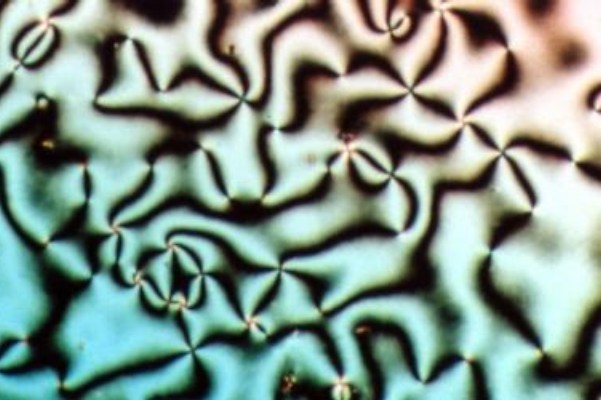
Liquid Crystal
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many types of LC phases, which can be distinguished by their optical properties (such as textures). The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. LC materials may not always be in a LC state of matter (just as water may be ice or water vapor). Liquid crystals can be divided into 3 main types: * thermotropic, *lyotropic, and * metallotropic. Thermotropic and lyotropic liquid crystals consist mostly of organic molecules, although a few minerals are also known. Thermotropic LCs exhibit a phase transition into the LC phase as temperature changes. Lyotropic LCs exhibit phase transitions as a function of b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spinodal Decomposition
Spinodal decomposition is a mechanism by which a single thermodynamic phase spontaneously separates into two phases (without nucleation). Decomposition occurs when there is no thermodynamic barrier to phase separation. As a result, phase separation via decomposition does not require the nucleation events resulting from thermodynamic fluctuations, which normally trigger phase separation. Spinodal decomposition is observed when mixtures of metals or polymers separate into two co-existing phases, each rich in one species and poor in the other. When the two phases emerge in approximately equal proportion (each occupying about the same volume or area), characteristic intertwined structures are formed that gradually coarsen (see animation). The dynamics of spinodal decomposition is commonly modeled using the Cahn–Hilliard equation. Spinodal decomposition is fundamentally different from nucleation and growth. When there is a nucleation barrier to the formation of a second phase, time i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |