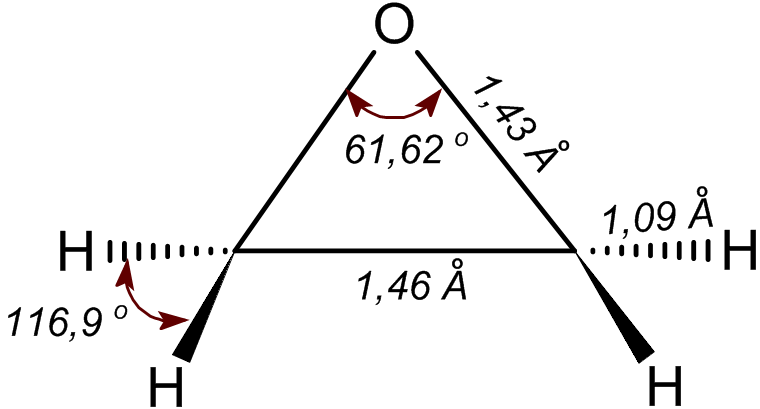
|
Poly(propylene Glycol) Diglycidyl Ether
Poly(propylene glycol) diglycidyl ether (PPGDGE) is an organic chemical in the glycidyl ether family. There are a number of variations depending on the starting molecular weight of the polypropylene glycol. They have the formula (C3H6O)n.C6H10O3 and the IUPAC name is Poly xy(methyl-1,2-ethanediyl)a-(2-oxiranylmethyl)-w-(2-oxiranylmethoxy)- A key use is as a modifier for epoxy resins as a reactive diluent and flexibilizer. It is REACH registered. Manufacturing The product is made by taking polypropylene glycol and epichlorohydrin and reacting in the presence of a Lewis acid catalyst to form a halohydrin. The next step is dehydrochlorination with sodium hydroxide. This forms the diglycidyl ether. Waste products are sodium chloride, water and excess sodium hydroxide ( alkaline brine). One of the quality control tests would involve measuring the epoxy value by determination of the epoxy equivalent weight. Use The molecule has 2 oxirane functionalities, and so a key use is modifying ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polypropylene Glycol
Polypropylene glycol or polypropylene oxide is the polymer (or macromolecule) of propylene glycol. Chemically it is a polyether, and, more generally speaking, it's a polyalkylene glycol (PAG) H S Code 3907.2000. The term polypropylene glycol or PPG is reserved for polymer of low- to medium-range molar mass when the nature of the end-group, which is usually a hydroxyl group, still matters. The term "oxide" is used for high-molar-mass polymer when end-groups no longer affect polymer properties. Between 60 and 70% of propylene oxide is converted to polyether polyols by the process called alkoxylation. Polymerization Polypropylene glycol is produced by ring-opening polymerization of propylene oxide. The initiator is an alcohol and the catalyst a base, usually potassium hydroxide. When the initiator is ethylene glycol or water the polymer is linear. With a multifunctional initiator like glycerine, pentaerythritol or sorbitol the polymer branches out. Conventional polymerization of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epoxy Value
Epoxy is the family of basic components or Curing (chemistry), cured end products of epoxy resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide functional group is also collectively called ''epoxy''. The IUPAC name for an epoxide group is an oxirane. Epoxy resins may be reacted (cross-linked) either with themselves through catalytic homopolymerisation, or with a wide range of co-reactants including polyfunctional amines, acids (and acid anhydrides), phenols, alcohols and thiols (usually called mercaptans). These co-reactants are often referred to as hardeners or curatives, and the cross-linking reaction is commonly referred to as curing. Reaction of polyepoxides with themselves or with polyfunctional hardeners forms a thermosetting polymer, often with favorable mechanical properties and high thermal and chemical resistance. Epoxy has a wide range of applications, including metal coatings, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycidol
Glycidol is an organic compound that contains both epoxide and alcohol functional groups. Being bifunctional, it has a variety of industrial uses. The compound is a slightly viscous liquid that is slightly unstable and is not often encountered in pure form. Synthesis and applications Glycidol is prepared by the epoxidation of allyl alcohol.Guenter Sienel, Robert Rieth, Kenneth T. Rowbottom "Epoxides" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. Glycidol is used as a stabilizer for natural oils and vinyl polymers and as a demulsifier. It is used as a chemical intermediate in the synthesis of glycerol, glycidyl ethers, esters and amines. It is used in surface coatings, chemical synthesis, pharmaceuticals, sanitary chemicals and sterilizing milk of magnesia, and as a gelation agent in solid propellants. #Alkylation of 2-methylquinazolin-4(3H)-one with glycidol affords diproqualone. #Dyphylline was made by the alkylation of theophylline with glycid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epoxide
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often volatile. Nomenclature A compound containing the epoxide functional group can be called an epoxy, epoxide, oxirane, and ethoxyline. Simple epoxides are often referred to as oxides. Thus, the epoxide of ethylene (C2H4) is ethylene oxide (C2H4O). Many compounds have trivial names; for instance, ethylene oxide is called "oxirane". Some names emphasize the presence of the epoxide functional group, as in the compound ''1,2-epoxyheptane'', which can also be called ''1,2-heptene oxide''. A polymer formed from epoxide precursors is called an ''epoxy'', but such materials do not contain epoxide groups (or contain only a few residual epoxy grou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toxicology
Toxicology is a scientific discipline, overlapping with biology, chemistry, pharmacology, and medicine, that involves the study of the adverse effects of chemical substances on living organisms and the practice of diagnosing and treating exposures to toxins and toxicants. The relationship between dose and its effects on the exposed organism is of high significance in toxicology. Factors that influence chemical toxicity include the dosage, duration of exposure (whether it is acute or chronic), route of exposure, species, age, sex, and environment. Toxicologists are experts on poisons and poisoning. There is a movement for evidence-based toxicology as part of the larger movement towards evidence-based practices. Toxicology is currently contributing to the field of cancer research, since some toxins can be used as drugs for killing tumor cells. One prime example of this is ribosome-inactivating proteins, tested in the treatment of leukemia. The word ''toxicology'' () is a neocla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Oxide
Ethylene oxide is an organic compound with the chemical formula, formula . It is a cyclic ether and the simplest epoxide: a three-membered Ring (chemistry), ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. Because it is a strained ring, ethylene oxide easily participates in a number of addition reactions that result in ring-opening. Ethylene oxide is isomeric with acetaldehyde and with vinyl alcohol. Ethylene oxide is industrially produced by oxidation of ethylene in the presence of silver catalyst. The reactivity that is responsible for many of ethylene oxide's hazards also makes it useful. Although too dangerous for direct household use and generally unfamiliar to consumers, ethylene oxide is used for making many consumer products as well as non-consumer chemicals and intermediates. These products include detergents, thickeners, solvents, plastics, and various organic chemicals such as ethylene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propylene Oxide
Propylene oxide is an acutely toxic and carcinogenic organic compound with the molecular formula CH3CHCH2O. This colourless volatile liquid with an odour similar to ether, is produced on a large scale industrially. Its major application is its use for the production of polyether polyols for use in making polyurethane plastics. It is a chiral epoxide, although it is commonly used as a racemic mixture. This compound is sometimes called 1,2-propylene oxide to distinguish it from its isomer 1,3-propylene oxide, better known as oxetane. Production Industrial production of propylene oxide starts from propylene. Two general approaches are employed, one involving hydrochlorination and the other involving oxidation. In 2005, about half of the world production was through chlorohydrin technology and one half via oxidation routes. The latter approach is growing in importance. Hydrochlorination route The traditional route proceeds via the conversion of propene to propylene chlorohydrin a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Waterborne Resins
Waterborne resins are sometimes called water-based resins. They are resins or polymeric resins that use water as the carrying medium as opposed to solvent or solvent-less. Resins are used in the production of coatings, adhesives, sealants, elastomers and composite materials. When the phrase waterborne resin is used, it usually describes all resins which have water as the main carrying solvent. The resin could be water soluble, water reducible or water dispersed. History Most coatings have four basic components. These are the resin, solvent, pigment and additive systems but the resin or binder is the key ingredient. Continuing environmental legislation in many countries along with geopolitics such as oil production are ensuring that chemists are increasingly turning to waterborne technology for paint/coatings and since resins or binders are the most important part of a coating, more of them are being developed and designed waterborne and there is a constantly increasing use by coa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sika AG
Sika AG is a Swiss multinational specialty chemical company that supplies to the building sector and motor vehicle industry, headquartered in Baar, Switzerland. The company develops and produces systems and products for bonding, sealing, damping, reinforcing, and protecting. Currently, it has over 27,000 employees, subsidiaries in more than 100 countries, and an annual sales turnover of CHF 9.3 billion. Sika was founded in 1910 when Kaspar Winkler laid the cornerstone for the firm. Winkler worked himself up from poverty to become a successful entrepreneur, and by the 1920s he was already engaged in founding subsidiaries abroad. Besides the brand Sika, more than 900 product brands are in use including Sikaflex, SikaTack, Sika ViscoCrete, SikaBond, Sikafloor, Sika CarboDur Sikagard, Sika MaxTack, Sikaplan, Sikament, Sikadur, SikaLastic, SikaRoof MTC, Sika Unitherm and Sika Sarnafil. In 2019, Sika acquired PAREX Group, a global company headquartered in France with more than 4,500 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elastomer
An elastomer is a polymer with viscoelasticity (i.e. both viscosity and elasticity) and with weak intermolecular forces, generally low Young's modulus and high failure strain compared with other materials. The term, a portmanteau of ''elastic polymer'', is often used interchangeably with rubber, although the latter is preferred when referring to vulcanisates. Each of the monomers which link to form the polymer is usually a compound of several elements among carbon, hydrogen, oxygen and silicon. Elastomers are amorphous polymers maintained above their glass transition temperature, so that considerable molecular reconformation is feasible without breaking of covalent bonds. At ambient temperatures, such rubbers are thus relatively compliant ( E ≈ 3 M Pa) and deformable. Their primary uses are for seals, adhesives and molded flexible parts. Application areas for different types of rubber are manifold and cover segments as diverse as tires, soles for shoes, and damping and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
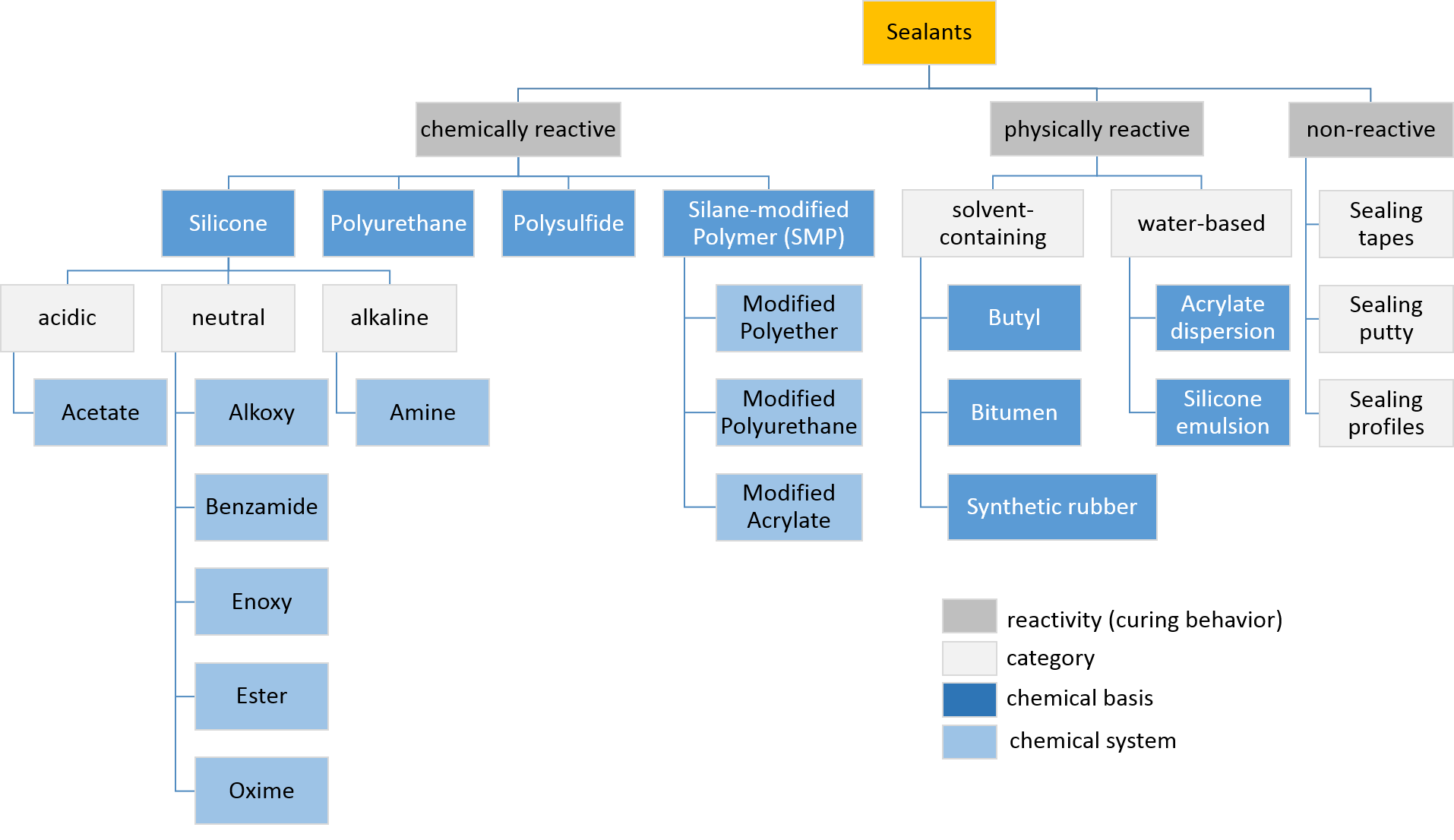
Sealant
Sealant is a substance used to block the passage of fluids through openings in materials, a type of mechanical seal. In building construction ''sealant'' is sometimes synonymous with ''caulking'' and also serve the purposes of blocking dust, sound and heat transmission. Sealants may be weak or strong, flexible or rigid, permanent or temporary. Sealants are not adhesives but some have adhesive qualities and are called ''adhesive-sealants'' or ''structural sealants''. History Sealants were first used in prehistory in the broadest sense as mud, grass and reeds to seal dwellings from the weather such as the daub in wattle and daub and thatching. Natural sealants and adhesive-sealants included plant resins such as pine pitch and birch pitch, bitumen, wax, tar, natural gum, clay (mud) mortar, lime mortar, lead, blood and egg. In the 17th century glazing putty was first used to seal window glass made with linseed oil and chalk, later other drying oils were also used to make oil-bas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adhesive
Adhesive, also known as glue, cement, mucilage, or paste, is any non-metallic substance applied to one or both surfaces of two separate items that binds them together and resists their separation. The use of adhesives offers certain advantages over other binding techniques such as sewing, mechanical fastenings, or welding. These include the ability to bind different materials together, the more efficient distribution of stress across a joint, the cost-effectiveness of an easily mechanized process, and greater flexibility in design. Disadvantages of adhesive use include decreased stability at high temperatures, relative weakness in bonding large objects with a small bonding surface area, and greater difficulty in separating objects during testing. Adhesives are typically organized by the method of adhesion followed by ''reactive'' or ''non-reactive'', a term which refers to whether the adhesive chemically reacts in order to harden. Alternatively, they can be organized eithe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_(6009043040).jpg)