|
Plaque Reduction Neutralization Test
The plaque reduction neutralization test is used to quantify the titer of neutralizing antibody for a virus. The serum sample or solution of antibody to be tested is diluted and mixed with a viral suspension. This is incubated to allow the antibody to react with the virus. This is poured over a confluent monolayer of host cells. The surface of the cell layer is covered in a layer of agar or carboxymethyl cellulose to prevent the virus from spreading indiscriminately. The concentration of plaque forming units can be estimated by the number of plaques (regions of infected cells) formed after a few days. Depending on the virus, the plaque forming units are measured by microscopic observation, fluorescent antibodies or specific dyes that react with infected cells. The concentration of serum to reduce the number of plaques by 50% compared to the serum free virus gives the measure of how much antibody is present or how effective it is. This measurement is denoted as the PRNT50 value. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titer
Titer (American English) or titre (British English) is a way of expressing concentration. Titer testing employs serial dilution to obtain approximate quantitative information from an analytical procedure that inherently only evaluates as positive or negative. The titre corresponds to the highest dilution factor that still yields a positive reading. For example, positive readings in the first 8 serial, twofold dilutions translate into a titer of 1:256 (i.e., 2−8). Titres are sometimes expressed by the denominator only, for example 1:256 is written 256. The term also has two other, conflicting meanings. In titration, the titer is the ratio of actual to nominal concentration of a titrant, e.g. a titer of 0.5 would require 1/0.5 = 2 times more titrant than nominal. This is to compensate for possible degradation of the titrant solution. Second, in textile engineering, titre is also a synonym for linear density. Etymology Titer has the same origin as the word "title", from the Fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibody
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the pathogen, called an antigen. Each tip of the "Y" of an antibody contains a paratope (analogous to a lock) that is specific for one particular epitope (analogous to a key) on an antigen, allowing these two structures to bind together with precision. Using this binding mechanism, an antibody can ''tag'' a microbe or an infected cell for attack by other parts of the immune system, or can neutralize it directly (for example, by blocking a part of a virus that is essential for its invasion). To allow the immune system to recognize millions of different antigens, the antigen-binding sites at both tips of the antibody come in an equally wide variety. In contrast, the remainder of the antibody is relatively constant. It only occurs in a few vari ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agar
Agar ( or ), or agar-agar, is a jelly-like substance consisting of polysaccharides obtained from the cell walls of some species of red algae, primarily from ogonori (''Gracilaria'') and "tengusa" (''Gelidiaceae''). As found in nature, agar is a mixture of two components, the linear polysaccharide agarose and a heterogeneous mixture of smaller molecules called agaropectin. It forms the supporting structure in the cell walls of certain species of algae and is released on boiling. These algae are known as agarophytes, belonging to the Rhodophyta (red algae) phylum. The processing of food-grade agar removes the agaropectin, and the commercial product is essentially pure agarose. Agar has been used as an ingredient in desserts throughout Asia and also as a solid substrate to contain culture media for microbiological work. Agar can be used as a laxative; an appetite suppressant; a vegan substitute for gelatin; a thickener for soups; in fruit preserves, ice cream, and other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxymethyl Cellulose
Carboxymethyl cellulose (CMC) or cellulose gum is a cellulose derivative with carboxymethyl groups (-CH2-COOH) bound to some of the hydroxyl groups of the glucopyranose monomers that make up the cellulose backbone. It is often used as its sodium salt, sodium carboxymethyl cellulose. It used to be marketed under the name Tylose, a registered trademark of SE Tylose. Preparation Carboxymethyl cellulose is synthesized by the alkali-catalyzed reaction of cellulose with chloroacetic acid. The polar (organic acid) carboxyl groups render the cellulose soluble and chemically reactive. Fabrics made of cellulose—e.g. cotton or viscose rayon—may also be converted into CMC. Following the initial reaction, the resultant mixture produces approximately 60% CMC and 40% salts ( sodium chloride and sodium glycolate); this product is the so-called technical CMC, which is used in detergents. An additional purification process is used to remove salts to produce pure CMC, which is used for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plaque Forming Units
A plaque-forming unit (PFU) is a measure used in virology to describe the number of virus particles capable of forming plaques per unit volume. It is a proxy measurement rather than a measurement of the absolute quantity of particles: viral particles that are defective or which fail to infect their target cell will not produce a plaque and thus will not be counted. For example, a solution of tick-borne encephalitis virus with a concentration of 1,000 PFU/μL indicates that 1 μL of the solution contains enough virus particles to produce 1000 infectious plaques in a cell mono-layer, but no inference can be made about the relationship of PFU to number of virus particles. The concept of plaque-forming units of virus is equivalent to the concept of colony-forming units of bacteria. See also *Virus quantification Virus quantification involves counting the number of viruses in a specific volume to determine the virus concentration. It is used in both research and development (R&D ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
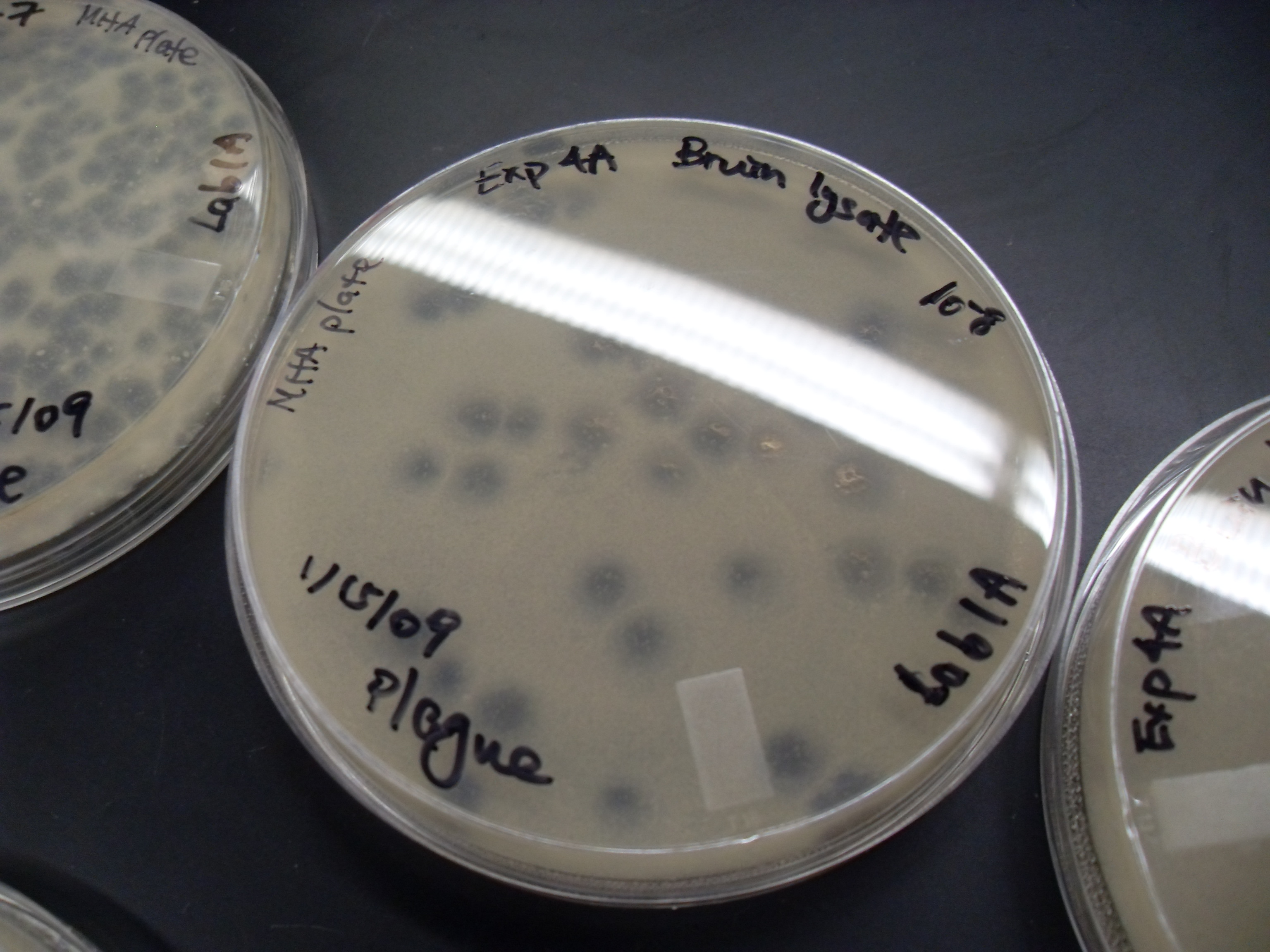
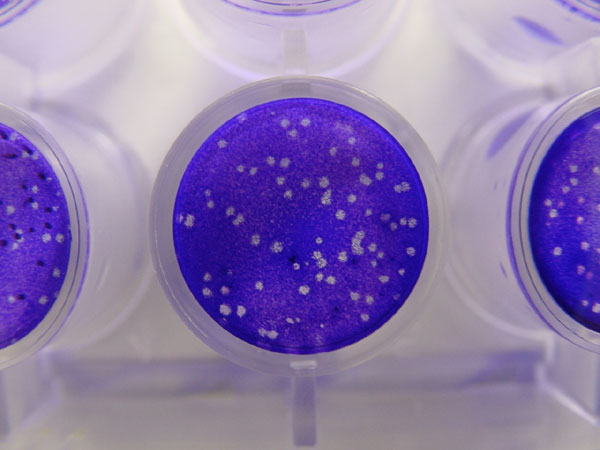
Viral Plaque
A viral plaque is a visible structure formed after introducing a viral sample to a cell culture grown on some nutrient medium. The virus will replicate and spread, generating regions of cell destruction known as plaques. For example, Vero cell or other tissue cultures may be used to investigate an influenza virus or coronavirus, while various bacterial cultures would be used for bacteriophages. Counting the number of plaques can be used as a method of virus quantification. These plaques can sometimes be detected visually using colony counters, in much the same way as bacterial colonies are counted; however, they are not always visible to the naked eye, and sometimes can only be seen through a microscope, or using techniques such as staining (e.g. neutral red for eukaryotes or giemsa for bacteria) or immunofluorescence. Special computer systems have been designed with the ability to scan samples in batches. The appearance of the plaque depends on the host strain, virus and the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sensitivity And Specificity
''Sensitivity'' and ''specificity'' mathematically describe the accuracy of a test which reports the presence or absence of a condition. Individuals for which the condition is satisfied are considered "positive" and those for which it is not are considered "negative". *Sensitivity (true positive rate) refers to the probability of a positive test, conditioned on truly being positive. *Specificity (true negative rate) refers to the probability of a negative test, conditioned on truly being negative. If the true condition can not be known, a " gold standard test" is assumed to be correct. In a diagnostic test, sensitivity is a measure of how well a test can identify true positives and specificity is a measure of how well a test can identify true negatives. For all testing, both diagnostic and screening, there is usually a trade-off between sensitivity and specificity, such that higher sensitivities will mean lower specificities and vice versa. If the goal is to return the ratio at w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemagglutination
Hemagglutination, or haemagglutination, is a specific form of agglutination that involves red blood cells (RBCs). It has two common uses in the laboratory: blood typing and the quantification of virus dilutions in a haemagglutination assay. Blood typing Blood type can be determined by using antibodies that bind to the A or B blood group antigens in a sample of blood. For example, if antibodies that bind the A blood group are added and agglutination occurs, the blood is either type A or type AB. To determine between type A or type AB, antibodies that bind the B group are added and if agglutination does not occur, the blood is type A. If agglutination does not occur with either antibodies that bind to type A or type B antigens, then neither antigen is present on the blood cells, which means the blood is type O. In blood grouping, the patient's serum is tested against RBCs of known blood groups and also the patient's RBCs are tested against known serum types. In this way the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
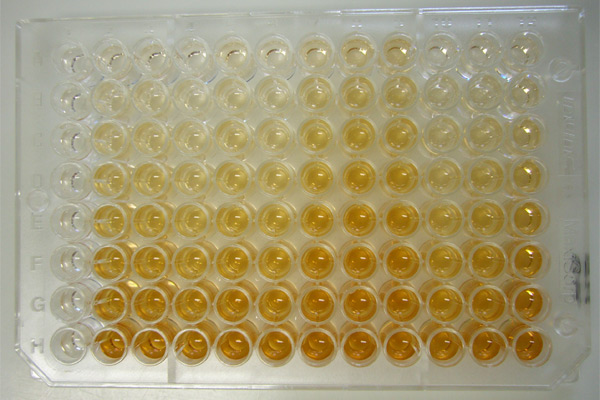
Enzyme Immunoassay
An enzyme immunoassay is any of several immunoassay methods that use an enzyme bound to an antigen or antibody. These may include: * Enzyme-linked immunosorbent assay The enzyme-linked immunosorbent assay (ELISA) (, ) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay uses a solid-phase type of enzyme immunoassay (EIA) to detect the presence ... (ELISA) * Enzyme multiplied immunoassay technique (EMIT) * Fluorescent enzyme immunoassays (FEIAs) * Chemiluminescent immunoassays (CLIAs) * Radioimmunoassays (RIAs) {{set index ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specificity (statistics)
''Sensitivity'' and ''specificity'' mathematically describe the accuracy of a test which reports the presence or absence of a condition. Individuals for which the condition is satisfied are considered "positive" and those for which it is not are considered "negative". *Sensitivity (true positive rate) refers to the probability of a positive test, conditioned on truly being positive. *Specificity (true negative rate) refers to the probability of a negative test, conditioned on truly being negative. If the true condition can not be known, a " gold standard test" is assumed to be correct. In a diagnostic test, sensitivity is a measure of how well a test can identify true positives and specificity is a measure of how well a test can identify true negatives. For all testing, both diagnostic and screening, there is usually a trade-off between sensitivity and specificity, such that higher sensitivities will mean lower specificities and vice versa. If the goal is to return the ratio at w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Virus Quantification
Virus quantification involves counting the number of viruses in a specific volume to determine the virus concentration. It is used in both research and development (R&D) in commercial and academic laboratories as well as production situations where the quantity of virus at various steps is an important variable. For example, the production of viral vaccines, recombinant proteins using viral vectors and viral antigens all require virus quantification to continually adapt and monitor the process in order to optimize production yields and respond to ever changing demands and applications. Examples of specific instances where known viruses need to be quantified include clone screening, multiplicity of infection (MOI) optimization and adaptation of methods to cell culture. This page discusses various techniques currently used to quantify viruses in liquid samples. These methods are separated into two categories, traditional vs. modern methods. Traditional methods are industry-stand ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunologic Tests
An immunoassay (IA) is a biochemical test that measures the presence or concentration of a macromolecule or a small molecule in a solution through the use of an antibody (usually) or an antigen (sometimes). The molecule detected by the immunoassay is often referred to as an "analyte" and is in many cases a protein, although it may be other kinds of molecules, of different sizes and types, as long as the proper antibodies that have the required properties for the assay are developed. Analytes in biological liquids such as serum or urine are frequently measured using immunoassays for medical and research purposes. Immunoassays come in many different formats and variations. Immunoassays may be run in multiple steps with reagents being added and washed away or separated at different points in the assay. Multi-step assays are often called separation immunoassays or heterogeneous immunoassays. Some immunoassays can be carried out simply by mixing the reagents and sample and making a phys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |