|
Photoelectron Diffraction
The photoelectric effect is the emission of electrons when electromagnetic radiation, such as light, hits a material. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physics, and solid state and quantum chemistry to draw inferences about the properties of atoms, molecules and solids. The effect has found use in electronic devices specialized for light detection and precisely timed electron emission. The experimental results disagree with classical electromagnetism, which predicts that continuous light waves transfer energy to electrons, which would then be emitted when they accumulate enough energy. An alteration in the intensity of light would theoretically change the kinetic energy of the emitted electrons, with sufficiently dim light resulting in a delayed emission. The experimental results instead show that electrons are dislodged only when the light exceeds a certain frequency—regardless of the light's intensity or du ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoelectric Effect In A Solid - Diagram
The photoelectric effect is the emission of electrons when electromagnetic radiation, such as light, hits a material. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physics, and Solid-state chemistry, solid state and quantum chemistry to draw inferences about the properties of atoms, molecules and solids. The effect has found use in Electronics, electronic devices specialized for light detection and precisely timed electron emission. The experimental results disagree with classical electromagnetism, which predicts that continuous light waves transfer energy to electrons, which would then be emitted when they accumulate enough energy. An alteration in the intensity (physics), intensity of light would theoretically change the kinetic energy of the emitted electrons, with sufficiently dim light resulting in a delayed emission. The experimental results instead show that electrons are dislodged only when the light exceeds a cert ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronvolt
In physics, an electronvolt (symbol eV, also written electron-volt and electron volt) is the measure of an amount of kinetic energy gained by a single electron accelerating from rest through an electric potential difference of one volt in vacuum. When used as a unit of energy, the numerical value of 1 eV in joules (symbol J) is equivalent to the numerical value of the charge of an electron in coulombs (symbol C). Under the 2019 redefinition of the SI base units, this sets 1 eV equal to the exact value Historically, the electronvolt was devised as a standard unit of measure through its usefulness in electrostatic particle accelerator sciences, because a particle with electric charge ''q'' gains an energy after passing through a voltage of ''V.'' Since ''q'' must be an integer multiple of the elementary charge ''e'' for any isolated particle, the gained energy in units of electronvolts conveniently equals that integer times the voltage. It is a common unit of ene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Introduction To Quantum Mechanics
Quantum mechanics is the study of matter and its interactions with energy on the scale of atomic and subatomic particles. By contrast, classical physics explains matter and energy only on a scale familiar to human experience, including the behavior of astronomical bodies such as the moon. Classical physics is still used in much of modern science and technology. However, towards the end of the 19th century, scientists discovered phenomena in both the large (macro) and the small ( micro) worlds that classical physics could not explain. The desire to resolve inconsistencies between observed phenomena and classical theory led to two major revolutions in physics that created a shift in the original scientific paradigm: the ''theory of relativity'' and the development of ''quantum mechanics''. This article describes how physicists discovered the limitations of classical physics and developed the main concepts of the quantum theory that replaced it in the early decades of the 20th ce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Compton Scattering
Compton scattering, discovered by Arthur Holly Compton, is the scattering of a high frequency photon after an interaction with a charged particle, usually an electron. If it results in a decrease in energy (increase in wavelength) of the photon (which may be an X-ray or gamma ray photon), it is called the Compton effect. Part of the energy of the photon is transferred to the recoiling electron. Inverse Compton scattering occurs when a charged particle transfers part of its energy to a photon. Introduction Compton scattering is an example of elastic scattering of light by a free charged particle, where the wavelength of the scattered light is different from that of the incident radiation. In Compton's original experiment (see Fig. 1), the energy of the X ray photon (≈17 keV) was significantly larger than the binding energy of the atomic electron, so the electrons could be treated as being free after scattering. The amount by which the light's wavelength changes is called t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Irradiated
Irradiation is the process by which an object is exposed to radiation. The exposure can originate from various sources, including natural sources. Most frequently the term refers to ionizing radiation, and to a level of radiation that will serve a specific purpose, rather than radiation exposure (radiation), exposure to normal levels of background radiation. The term irradiation usually excludes the exposure to non-ionizing radiation, such as infrared, visible light, microwaves from cellular phones or electromagnetic waves emitted by radio and TV receivers and power supplies. Applications Sterilization If administered at appropriate levels, all forms of ionizing radiation can sterilization (microbiology), sterilize objects, including medical instruments, disposables such as syringes, and Food irradiation, sterilize food. Ionizing radiation (electron beam processing, electron beams, X-rays and gamma rays) may be used to kill bacteria in food or other organic material, including ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Binding Energy
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron's mass is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum ( spin) of a half-integer value, expressed in units of the reduced Planck constant, . Being fermions, no two electrons can occupy the same quantum state, per the Pauli exclusion principle. Like all elementary particles, electrons exhibit properties of both particles and waves: They can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer de Brogl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photon Energy
Photon energy is the energy carried by a single photon. The amount of energy is directly proportional to the photon's electromagnetic frequency and thus, equivalently, is inversely proportional to the wavelength. The higher the photon's frequency, the higher its energy. Equivalently, the longer the photon's wavelength, the lower its energy. Photon energy can be expressed using any unit of energy. Among the units commonly used to denote photon energy are the electronvolt (eV) and the joule (as well as its multiples, such as the microjoule). As one joule equals 6.24 × 1018 eV, the larger units may be more useful in denoting the energy of photons with higher frequency and higher energy, such as gamma rays, as opposed to lower energy photons as in the optical and radio frequency regions of the electromagnetic spectrum. Formulas Physics Photon energy is directly proportional to frequency. E = hf where *E is energy *h is the Planck constant * f is frequency This equation is know ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
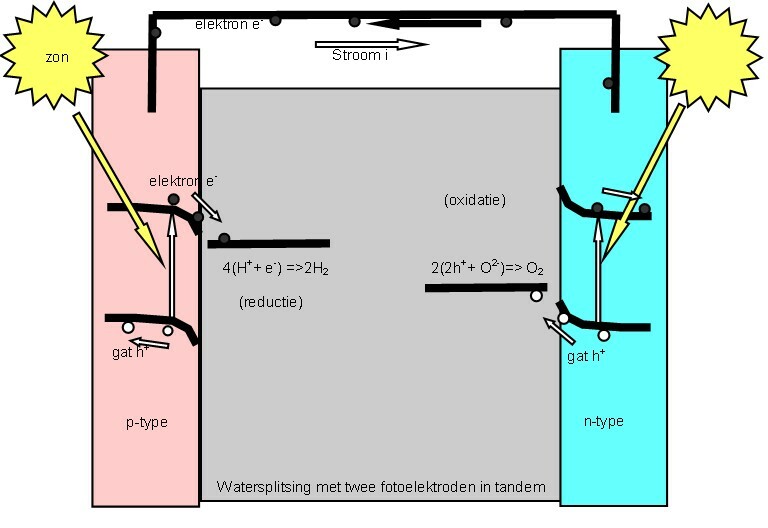
Photoelectrochemical Cell
A "photoelectrochemical cell" is one of two distinct classes of device. The first produces electrical energy similarly to a dye-sensitized photovoltaic cell, which meets the standard definition of a photovoltaic cell. The second is a photoelectrolytic cell, that is, a device which uses light incident on a photosensitizer, semiconductor, or aqueous metal immersed in an electrolytic solution to directly cause a chemical reaction, for example to produce hydrogen via the electrolysis of water. Both types of device are varieties of solar cell, in that a photoelectrochemical cell's function is to use the photoelectric effect (or, very similarly, the photovoltaic effect) to convert electromagnetic radiation (typically sunlight) either directly into electrical power, or into something which can itself be easily used to produce electrical power (hydrogen, for example, can be burned to create electrical power, see photohydrogen). Two principles The standard photovoltaic effect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photovoltaic Effect
The photovoltaic effect is the generation of voltage and electric current in a material upon exposure to light. It is a physical and chemical phenomenon. The photovoltaic effect is closely related to the photoelectric effect. For both phenomena, light is absorbed, causing excitation of an electron or other charge carrier to a higher-energy state. The main distinction is that the term ''photoelectric effect'' is now usually used when the electron is ejected out of the material (usually into a vacuum) and ''photovoltaic effect'' used when the excited charge carrier is still contained within the material. In either case, an electric potential (or voltage) is produced by the separation of charges, and the light has to have a sufficient energy to overcome the potential barrier for excitation. The physical essence of the difference is usually that photoelectric emission separates the charges by ballistic conduction and photovoltaic emission separates them by diffusion, but some "hot c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoconductivity
Photoconductivity is an optical and electrical phenomenon in which a material becomes more electrically conductive due to the absorption of electromagnetic radiation such as visible light, ultraviolet light, infrared light, or gamma radiation. When light is absorbed by a material such as a semiconductor, the number of free electrons and holes increases, resulting in increased electrical conductivity. To cause excitation, the light that strikes the semiconductor must have enough energy to raise electrons across the band gap, or to excite the impurities within the band gap. When a bias voltage and a load resistor are used in series with the semiconductor, a voltage drop across the load resistors can be measured when the change in electrical conductivity of the material varies the current through the circuit. Classic examples of photoconductive materials include: * photographic film: Kodachrome, Fujifilm, Agfachrome, Ilford, ''etc.'', based on silver sulfide and silver bromide. * th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saunders (imprint)
Saunders is an American academic publisher based in the United States. It is currently an imprint of Elsevier. Formerly independent, the W. B. Saunders company was acquired by CBS in 1968, who added it to their publishing division Holt, Rinehart & Winston. When CBS left the publishing field in 1986, it sold the academic publishing units to Harcourt Brace Jovanovich. Harcourt was acquired by Reed Elsevier RELX plc (pronounced "Rel-ex") is a British multinational information and analytics company headquartered in London, England. Its businesses provide scientific, technical and medical information and analytics; legal information and analytics; ... in 2001. . Northern Il ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wave–particle Duality
Wave–particle duality is the concept in quantum mechanics that every particle or quantum entity may be described as either a particle or a wave. It expresses the inability of the classical concepts "particle" or "wave" to fully describe the behaviour of quantum-scale objects. As Albert Einstein wrote: Through the work of Max Planck, Albert Einstein, Louis de Broglie, Arthur Compton, Niels Bohr, Erwin Schrödinger and many others, current scientific theory holds that all particles exhibit a wave nature and vice versa. This phenomenon has been verified not only for elementary particles, but also for compound particles like atoms and even molecules. For macroscopic particles, because of their extremely short wavelengths, wave properties usually cannot be detected. Although the use of the wave–particle duality has worked well in physics, the meaning or interpretation has not been satisfactorily resolved; see interpretations of quantum mechanics. Bohr regarded the " ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |