|
Phorone
Phorone, or diisopropylidene acetone, is a yellow crystalline substance with a geranium odor, with formula or . Preparation It was first obtained in 1837 in impure form by the French chemist Auguste Laurent, who called it "camphoryle". In 1849, the French chemist Charles Frédéric Gerhardt and his student Jean Pierre Liès-Bodart prepared it in a pure state and named it "phorone". On both occasions it was produced by ketonization through the dry distillation of the calcium salt of camphoric acid. : It is now typically obtained by the acid-catalysed twofold aldol condensation of three molecules of acetone. Mesityl oxide is obtained as an intermediate and can be isolated. Crude phorone can be purified by repeated recrystallization from ethanol or ether, in which it is soluble. Reactions Phorone can condense with ammonia to form triacetone amine. See also *Isophorone Isophorone is an α,β-unsaturated cyclic ketone. It is a colorless liquid with a characteristic peppe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mesityl Oxide
Mesityl oxide is a Carbonyl#.CE.B1.2C.CE.B2-Unsaturated_carbonyl_compounds, α,β-unsaturated ketone with the formula CH3C(O)CH=C(CH3)2. This compound is a colorless, volatile liquid with a honey-like odor. Synthesis It is prepared by the aldol condensation of acetone to give diacetone alcohol, which readily dehydrates to give this compound. : Phorone and isophorone may be formed under the same conditions. Isophorone originates via a Michael addition: : Phorone is formed by continued aldol condensation: : Uses Mesityl oxide is used as a solvent and in the production of methyl isobutyl ketone by hydrogenation: : Further hydrogenation gives 4-methyl-2-pentanol (methyl isobutyl carbinol). Dimedone is another established use of mesityl oxide. References {{reflist External linksIPCS INCHEM Description Enones Ketone solvents ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charles Frédéric Gerhardt
Charles Frédéric Gerhardt (21 August 1816 – 19 August 1856) was a French chemist, born in Alsace and active in Paris, Montpellier, and his native Strasbourg. Biography He was born in Strasbourg, which is where he attended the gymnasium (an advanced academic secondary school). He then studied at the Karlsruhe Institute of Technology, where Friedrich Walchner's lectures first stimulated his interest in chemistry. Next he attended the school of commerce in Leipzig, where he studied chemistry under Otto Linné Erdmann, who further developed his interest into a passion for questions of speculative chemistry. Returning home in 1834, he entered his father's white lead factory, but soon found that business was not to his liking, and after a sharp disagreement with his father in his 20th year he enlisted in a cavalry regiment. In a few months military life became equally distasteful, and he purchased his discharge with the assistance of the German chemist Justus von Liebig. After a s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isophorone
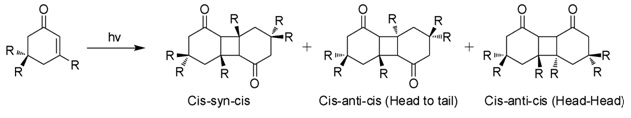
Isophorone is an Alpha-beta Unsaturated carbonyl compounds, α,β-unsaturated cyclic ketone. It is a colorless liquid with a characteristic peppermint-like odor, although commercial samples can appear yellowish. Used as a solvent and as a precursor to polymers, it is produced on a large scale industrially. Structure and reactivity Isophorone undergoes reactions characteristic of an α,β-unsaturated ketone. Hydrogenation gives the cyclohexanone derivative. Epoxidation with basic hydrogen peroxide affords the oxide. Isophorone is degraded by attack of hydroxyl radicals. Photodimerization When exposed to sunlight in aqueous solutions, isophorone undergoes 2+2 photocycloaddition to give three isomeric photodimers (Figure). These "diketomers" are cis-syn-cis, head to tail (HT), cys-anti-cys (HT), and head-head (HH). The formation of HH photodimers is favored over HT photodimers with increasing polarity of the medium. Natural Occurrence Isophorone occurs naturally in cran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pelargonium
''Pelargonium'' () is a genus of flowering plants that includes about 280 species of perennials, succulents, and shrubs, commonly called geraniums, pelargoniums, or storksbills. '' Geranium'' is also the botanical name and common name of a separate genus of related plants, also known as cranesbills. Both genera belong to the family Geraniaceae. Carl Linnaeus originally included all the species in one genus, ''Geranium'', and they were later separated into two genera by Charles Louis L'Héritier de Brutelle in 1789. While ''Geranium'' species are mostly temperate herbaceous plants, dying down in winter, ''Pelargonium'' species are evergreen perennials indigenous to warm temperate and tropical regions of the world, with many species in southern Africa. They are drought and heat tolerant, but can tolerate only minor frosts. Some species are extremely popular garden plants, grown as houseplants and bedding plants in temperate regions. They have a long flowering period, with flowers m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include Subscript and superscript, subscripts and superscripts. A chemical formula is not a chemical nomenclature, chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called ''empirical formulae'', which use letters and numbers ind ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Auguste Laurent
Auguste Laurent (14 November 1807 – 15 April 1853) was a French chemist who helped in the founding of organic chemistry with his discoveries of anthracene, phthalic acid, and carbolic acid. He devised a systematic nomenclature for organic chemistry based on structural grouping of atoms within molecules to determine how the molecules combine in organic reactions. He studied under Jean-Baptiste Dumas as a laboratory assistant and worked with Charles Frédéric Gerhardt. He died in Paris from tuberculosis. Bibliography Marc Tiffeneau (ed.) (1918). ''Correspondance de Charles Gerhardt'', tome 1, ''Laurent et Gerhardt'', Paris, Masson. References * Fisher, Nicholas W. "Auguste Laurent." Encyclopædia Britannica Mobile. 2013. web. External links * http://scienceworld.wolfram.com/biography/Laurent.html * Friedrich August Kekulé von Stradonitz Friedrich may refer to: Names * Friedrich (surname), people with the surname ''Friedrich'' * Friedrich (given name), people with the give ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
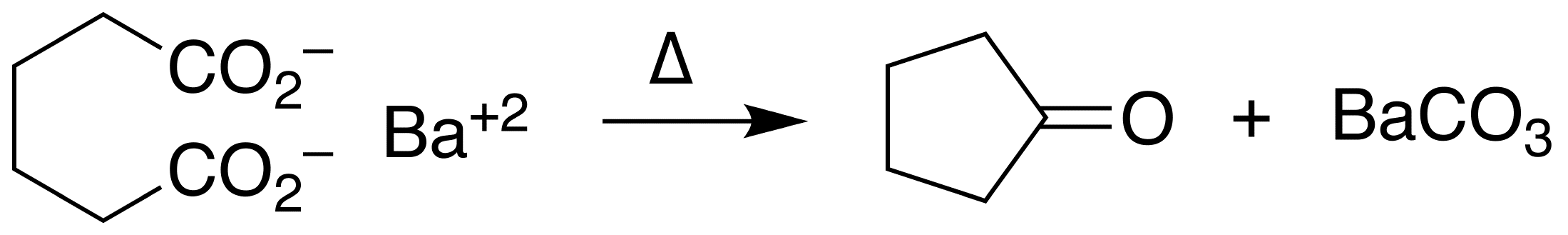
Ketonization
In organic chemistry, ketonic decarboxylation (also known as decarboxylative ketonization) is a type of organic reaction and a decarboxylation converting two equivalents of a carboxylic acid () to a symmetric ketone () by the application of heat with expulsion of one equivalent of water () and one equivalent of carbon dioxide (): :\ce\mathbf + \ce\mathbf \longrightarrow \ce\mathbf + \ce Bases promote this reaction. The reaction mechanism likely involves nucleophilic attack of the alpha-carbon of one acid group on the other acid group's carbonyl (), possibly as a concerted reaction with the decarboxylation. The initial formation of an intermediate carbanion via decarboxylation of one of the acid groups prior to the nucleophilic attack has been proposed, but is unlikely since the byproduct resulting from the carbanion's protonation by the acid has never been reported. This reaction is different from oxidative decarboxylation, which proceeds through a radical mechanism and is charact ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Camphoric Acid
Camphoric acid, C10 H16 O4 or in Latin form Acidum camphoricum, is a white crystallisable substance obtained from the oxidation of camphor. It exists in three optically different forms; the dextrorotatory one is obtained by the oxidation of dextrorotatory camphor and is used in pharmaceuticals. History Acidum camphoricum was studied and isolated for the first time by French pharmacist Nicolas Vauquelin in the early 19th century but it wasn't until September 1874 that Dutch chemist Jacobus H. van 't Hoff proposed the first suggestion for its molecular structure and optical properties. Haller and Blanc synthesized camphor from camphoric acid. In 1904, Finnish chemist Gustav Komppa became the first to succeed in manufacturing synthetic camphoric acid from diethyl oxalate and 3,3-dimethylpentanoic acid, and thus proving the structure of camphor. Chemical properties and isolation Camphoric acid may be prepared by oxidising camphor with nitric acid Nitric acid is the inorgani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldol Condensation
An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties (of aldehydes or ketones) react to form a β-hydroxyaldehyde or β-hydroxyketone (an aldol reaction), and this is then followed by dehydration to give a conjugated enone. The overall reaction is as follows (where the Rs can be H): Aldol condensations are important in organic synthesis and biochemistry as ways to form carbon–carbon bonds. In its usual form, it involves the nucleophilic addition of a ketone enolate to an aldehyde to form a β-hydroxy ketone, or "aldol" (aldehyde + alcohol), a structural unit found in many naturally occurring molecules and pharmaceuticals. The term ''aldol condensation'' is also commonly used, especially in biochemistry, to refer to just the first (addition) stage of the process—the aldol reaction itself—as catalyzed by aldolases. However, this is formally an addition reaction rather than a condensation reaction because it does not invo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour. Acetone is miscible with water and serves as an important organic solvent in its own right, in industry, home, and laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and production of methyl methacrylate (and from that PMMA) as well as bisphenol A.Acetone World Petrochemicals report, January 2010Stylianos Sifniades, Alan B. Levy, "Acetone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. It is a common building block in |
Phoron Formation
Tempering is a cooking technique used in India, Bangladesh, Nepal, Pakistan and Sri Lanka, in which whole spices (and sometimes also other ingredients such as dried chillies, minced ginger root or sugar) are roasted briefly in oil or ghee to liberate essential oils from cells and thus enhance their flavours, before being poured, together with the oil, into a dish. Tempering is also practiced by dry-roasting whole spices in a pan before grinding the spices. Tempering is typically done at the beginning of cooking, before adding the other ingredients for a curry or similar dish, or it may be added to a dish at the end of cooking, just before serving (as with a dal, sambar or stew). Ingredients used Ingredients typically used in tempering include cumin seeds, black mustard seeds, fennel seeds, '' kalonji'', fresh green chilis, dried red chilis, fenugreek seeds, asafoetida, cassia, cloves, urad dal, curry leaves, chopped onion, garlic, or tejpat leaves. When using multiple ingred ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is both caust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


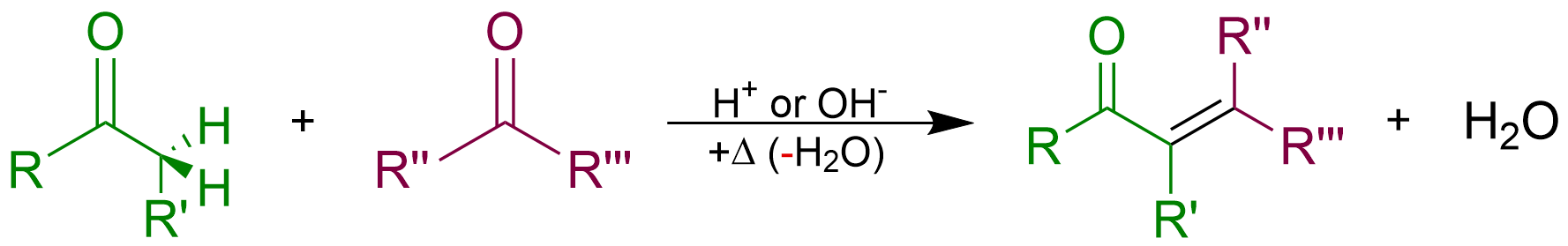


-3D-balls.png)