|
Philippinite
Philippinites, or rizalites are tektites found in the Philippines. They are considered to be about 710,000 years old on the average and generally ranging in size from millimeters to centimeters. Their age corresponds with the age of other tektites in the Australian strewn tektite field. In 1964, a very large philippinite, weighing with dimensions 6.5 x 6.2 x 5.2 cm, was purchased by the University of California, Los Angeles Department of Astronomy. The heaviest philippinite ever found weighs in its splash-form, which is also the heaviest tektite of this kind. Etymology The term rizalite was named after the Philippine province of Rizal where the first black tektites were rediscovered in October 1926 at Novaliches (which was then part of Rizal). Although, it was only in 1928 that the term was proposed by American anthropologist H. Otley Beyer, dubbed as the father of Philippine tektite studies, to refer to tektites found in the Philippines. Philippinite has become the more fav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Philippinite
Philippinites, or rizalites are tektites found in the Philippines. They are considered to be about 710,000 years old on the average and generally ranging in size from millimeters to centimeters. Their age corresponds with the age of other tektites in the Australian strewn tektite field. In 1964, a very large philippinite, weighing with dimensions 6.5 x 6.2 x 5.2 cm, was purchased by the University of California, Los Angeles Department of Astronomy. The heaviest philippinite ever found weighs in its splash-form, which is also the heaviest tektite of this kind. Etymology The term rizalite was named after the Philippine province of Rizal where the first black tektites were rediscovered in October 1926 at Novaliches (which was then part of Rizal). Although, it was only in 1928 that the term was proposed by American anthropologist H. Otley Beyer, dubbed as the father of Philippine tektite studies, to refer to tektites found in the Philippines. Philippinite has become the more fav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tektite
Tektites (from grc, τηκτός , meaning 'molten') are gravel-sized bodies composed of black, green, brown or grey natural glass formed from terrestrial debris ejected during meteorite impacts. The term was coined by Austrian geologist Franz Eduard Suess (1867–1941), son of Eduard Suess. They generally range in size from millimetres to centimetres. Millimetre-scale tektites are known as microtektites.French, B. M. (1998) ''Traces of Catastrophe: A Handbook of Shock-Metamorphic Effects in Terrestrial Meteorite Impact Structures.'' LPI Contribution No. 954. Lunar and Planetary Institute, Houston, Texas. 120 pp.McCall, G. J. H. (2001) ''Tektites in the Geological Record: Showers of Glass from the Sky.'' The Geological Society Publishing House, Bath, United Kingdom. 256 pp. Montanari, A., and C. Koeberl (2000) ''Impact Stratigraphy. The Italian Record.'' Lecture Notes in Earth Sciences Series no. 93. Springer-Verlag, New York, New York. 364 pp. Tektites are characterized by: # ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Encyclopedia Britannica
An encyclopedia (American English) or encyclopædia (British English) is a reference work or compendium providing summaries of knowledge either general or special to a particular field or discipline. Encyclopedias are divided into articles or entries that are arranged alphabetically by article name or by thematic categories, or else are hyperlinked and searchable. Encyclopedia entries are longer and more detailed than those in most dictionaries. Generally speaking, encyclopedia articles focus on '' factual information'' concerning the subject named in the article's title; this is unlike dictionary entries, which focus on linguistic information about words, such as their etymology, meaning, pronunciation, use, and grammatical forms.Béjoint, Henri (2000)''Modern Lexicography'', pp. 30–31. Oxford University Press. Encyclopedias have existed for around 2,000 years and have evolved considerably during that time as regards language (written in a major international or a verna ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Pentoxide
Phosphorus pentoxide is a chemical compound with molecular formula P4 O10 (with its common name derived from its empirical formula, P2O5). This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant and dehydrating agent. Structure Phosphorus pentoxide crystallizes in at least four forms or polymorphs. The most familiar one, a metastable form (shown in the figure), comprises molecules of P4O10. Weak van der Waals forces hold these molecules together in a hexagonal lattice (However, in spite of the high symmetry of the molecules, the crystal packing is not a close packing). The structure of the P4O10 cage is reminiscent of adamantane with ''T''d symmetry point group. It is closely related to the corresponding anhydride of phosphorous acid, P4O6. The latter lacks terminal oxo groups. Its density is 2.30 g/cm3. It boils at 423 °C under atmospheric pressure; if heated more rapidly it can sublimate. This form can be made by condensing the vap ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
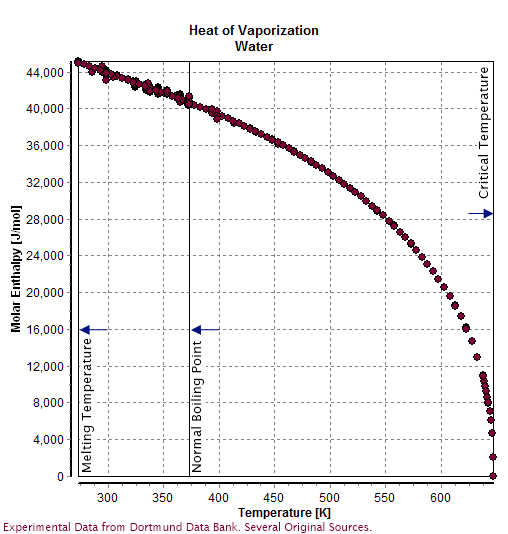
Properties Of Water
Water () is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an inherent hint of blue. It is by far the most studied chemical compound and is described as the "universal solvent" and the "solvent of life". It is the most abundant substance on the surface of Earth and the only common substance to exist as a solid, liquid, and gas on Earth's surface. It is also the third most abundant molecule in the universe (behind molecular hydrogen and carbon monoxide). Water molecules form hydrogen bonds with each other and are strongly polar. This polarity allows it to dissociate ions in salts and bond to other polar substances such as alcohols and acids, thus dissolving them. Its hydrogen bonding causes its many unique properties, such as having a solid form less dense than its liquid form, a relatively high boiling point of 100 °C for its molar mass, and a high heat capacity. Water is amphoteric, meani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Oxide
Potassium oxide ( K O) is an ionic compound of potassium and oxygen. It is a base. This pale yellow solid is the simplest oxide of potassium. It is a highly reactive compound that is rarely encountered. Some industrial materials, such as fertilizers and cements, are assayed assuming the percent composition that would be equivalent to K2O. Production Potassium oxide is produced from the reaction of oxygen and potassium; this reaction affords potassium peroxide, K2O2. Treatment of the peroxide with potassium produces the oxide: : K2O2 + 2 K -> 2 K2O Alternatively and more conveniently, K2O is synthesized by heating potassium nitrate with metallic potassium: :2KNO3 + 10K -> 6K2O + N2 (^) Other possibility is to heat potassium peroxide at 500 °C which decomposes at that temperature giving pure potassium oxide and oxygen. :2K2O2 -> 2K2O + O2 (^) Potassium hydroxide cannot be further dehydrated to the oxide but it can react with molten potassium to produce it, releasing hydr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Oxide
Sodium oxide is a chemical compound with the formula Na2 O. It is used in ceramics and glasses. It is a white solid but the compound is rarely encountered. Instead "sodium oxide" is used to describe components of various materials such as glasses and fertilizers which contain oxides that include sodium and other elements. Structure The structure of sodium oxide has been determined by X-ray crystallography. Most alkali metal oxides M2O (M = Li, Na, K, Rb) crystallise in the antifluorite structure. In this motif the positions of the anions and cations are reversed relative to their positions in CaF2, with sodium ions tetrahedrally coordinated to 4 oxide ions and oxide cubically coordinated to 8 sodium ions. Preparation Sodium oxide is produced by the reaction of sodium with sodium hydroxide, sodium peroxide, or sodium nitrite: : 2 NaOH + 2 Na → 2 Na2O + H2 To the extent that NaOH is contaminated with water, correspondingly greater amounts of sodium are employed. Excess s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Oxide
Magnesium oxide ( Mg O), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions held together by ionic bonding. Magnesium hydroxide forms in the presence of water (MgO + H2O → Mg(OH)2), but it can be reversed by heating it to remove moisture. Magnesium oxide was historically known as magnesia alba (literally, the white mineral from Magnesia), to differentiate it from ''magnesia negra'', a black mineral containing what is now known as manganese. Related oxides While "magnesium oxide" normally refers to MgO, the compound magnesium peroxide MgO2 is also known. According to evolutionary crystal structure prediction, MgO2 is thermodynamically stable at pressures above 116 GPa (gigapascals), and a semiconducting suboxide Mg3O2 is thermodynamically stable above 500 GPa. Because of its stability, MgO is used as a model sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Oxide
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, Caustic (substance), caustic, alkaline, crystalline solid at room temperature. The broadly used term "''lime (material), lime''" connotes calcium-containing inorganic materials, in which carbonates, oxides and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate. By contrast, ''quicklime'' specifically applies to the single chemical compound calcium oxide. Calcium oxide that survives processing without reacting in building products such as cement is called free lime. Quicklime is relatively inexpensive. Both it and a chemical derivative (calcium hydroxide, of which quicklime is the base anhydride) are important commodity chemicals. Preparation Calcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate (CaCO3; mineral calcite) in a lime kiln. This is accomplished by hea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese(II) Oxide
Manganese(II) oxide is an inorganic compound with chemical formula MnO.Arno H. Reidies "Manganese Compounds" Ullmann's Encyclopedia of Chemical Technology 2007; Wiley-VCH, Weinheim. It forms green crystals. The compound is produced on a large scale as a component of fertilizers and food additives. Structure, stoichiometry, reactivity Like many monoxides, MnO adopts the rock salt structure, where cations and anions are both octahedrally coordinated. Also like many oxides, manganese(II) oxide is often nonstoichiometric: its composition can vary from MnO to MnO1.045. Below 118 K MnO is antiferromagnetic. MnO has the distinction of being one of the first compounds to have its magnetic structure determined by neutron diffraction, the report appearing in 1951. This study showed that the Mn2+ ions form a face centered cubic magnetic sub-lattice where there are ferromagnetically coupled sheets that are anti-parallel with adjacent sheets. Manganese(II) oxide undergoes the chemical r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(II) Oxide
Iron(II) oxide or ferrous oxide is the inorganic compound with the formula FeO. Its mineral form is known as wüstite. One of several iron oxides, it is a black-colored powder that is sometimes confused with rust, the latter of which consists of hydrated iron(III) oxide (ferric oxide). Iron(II) oxide also refers to a family of related non-stoichiometric compounds, which are typically iron deficient with compositions ranging from Fe0.84O to Fe0.95O. Preparation FeO can be prepared by the thermal decomposition of iron(II) oxalate. :FeC2O4 → FeO + CO2 + CO The procedure is conducted under an inert atmosphere to avoid the formation of iron(III) oxide (Fe2O3). A similar procedure can also be used for the synthesis of manganous oxide and stannous oxide. Stoichiometric FeO can be prepared by heating Fe0.95O with metallic iron at 770 °C and 36 kbar.Wells A.F. (1984) ''Structural Inorganic Chemistry'' 5th edition Oxford University Press Reactions FeO is therm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(III) Oxide
Iron(III) oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron(II) oxide (FeO), which is rare; and iron(II,III) oxide (Fe3O4), which also occurs naturally as the mineral magnetite. As the mineral known as hematite, Fe2O3 is the main source of iron for the steel industry. Fe2O3 is readily attacked by acids. Iron(III) oxide is often called rust, and to some extent this label is useful, because rust shares several properties and has a similar composition; however, in chemistry, rust is considered an ill-defined material, described as Hydrous ferric oxide. Structure Fe2O3 can be obtained in various polymorphs. In the main one, α, iron adopts octahedral coordination geometry. That is, each Fe center is bound to six oxygen ligands. In the γ polymorph, some of the Fe sit on tetrahedral sites, with four oxygen ligands. Alpha phase α-Fe2O3 has the rhombohedral, corundum (α-Al2O3) structure and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |