|
Pharmacotoxicology
Pharmacotoxicology entails the study of the consequences of toxic exposure to pharmaceutical drugs and agents in the health care field. The field of pharmacotoxicology also involves the treatment and prevention of pharmaceutically induced side effects. Pharmacotoxicology can be separated into two different categories: pharmacodynamics (the effects of a drug on an organism), and pharmacokinetics (the effects of the organism on the drug). Mechanisms of Pharmaceutical Drug Toxicity There are many mechanisms by which pharmaceutical drugs can have toxic implications. A very common mechanism is covalent binding of either the drug or its metabolites to specific enzymes or receptor in tissue-specific pathways that then will elicit toxic responses. Covalent binding can occur during both on-target and off-target situations and after biotransformation. On-target toxicity. On-target toxicity is also referred to as mechanism-based toxicity. This type of adverse effect that results from pharmac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adverse Effect (medicine)
An adverse effect is an undesired harmful effect resulting from a medication or other intervention, such as surgery. An adverse effect may be termed a "side effect", when judged to be secondary to a main or therapeutic effect. The term complication is similar to adverse effect, but the latter is typically used in pharmacological contexts, or when the negative effect is expected or common. If the negative effect results from an unsuitable or incorrect dosage or procedure, this is called a medical error and not an adverse effect. Adverse effects are sometimes referred to as "iatrogenic" because they are generated by a physician/treatment. Some adverse effects occur only when starting, increasing or discontinuing a treatment. Adverse effects can also be caused by placebo treatments (in which case the adverse effects are referred to as nocebo effects). Using a drug or other medical intervention which is contraindicated may increase the risk of adverse effects. Adverse effects may c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toxin
A toxin is a naturally occurring organic poison produced by metabolic activities of living cells or organisms. Toxins occur especially as a protein or conjugated protein. The term toxin was first used by organic chemist Ludwig Brieger (1849–1919) and is derived from the word toxic. Toxins can be small molecules, peptides, or proteins that are capable of causing disease on contact with or absorption by body tissues interacting with biological macromolecules such as enzymes or cellular receptors. Toxins vary greatly in their toxicity, ranging from usually minor (such as a bee sting) to potentially fatal even at extremely low doses (such as botulinum toxin). Toxins are largely secondary metabolites, which are organic compounds that are not directly involved in an organism's growth, development, or reproduction, instead often aiding it in matters of defense. Terminology Toxins are often distinguished from other chemical agents strictly based on their biological origin. Le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactive Oxygen Species
In chemistry, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (). Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen. The reduction of molecular oxygen () produces superoxide (), which is the precursor to most other reactive oxygen species: :O2 + e^- -> \ ^\bullet O2- Dismutation of superoxide produces hydrogen peroxide (): :2 H+ + \ ^\bullet O2^- + \ ^\bullet O2^- -> H2O2 + O2 Hydrogen peroxide in turn may be partially reduced, thus forming hydroxide ions and hydroxyl radicals (), or fully reduced to water: :H2O2 + e^- -> HO^- + \ ^\bullet OH :2 H+ + 2 e- + H2O2 -> 2 H2O In a biological context, ROS are byproducts of the normal metabolism of oxygen. ROS have roles in cell signaling and homeostasis. ROS are intrinsic to cellular functioning, and are present at low and stationary levels in normal cells. In plants, ROS are involved in metabolic processes related to photoprotection and toleran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Taxanes
Taxanes are a class of diterpenes. They were originally identified from plants of the genus ''Taxus'' (yews), and feature a taxadiene core. Paclitaxel (Taxol) and docetaxel (Taxotere) are widely used as chemotherapy agents. Cabazitaxel was FDA approved to treat hormone-refractory prostate cancer. Taxanes present difficulties in formulation as medicines because they are poorly soluble in water. Production As their name suggests, taxanes were first derived from natural sources, but some have been semisynthesized. Paclitaxel was originally derived from the Pacific yew tree. Taxanes are difficult to synthesize because of their numerous chiral centres—taxol has 11 of these. Recently, the presence of taxanes in the shells and leaves of ''Corylus avellana'' (the common hazel plant) has been reported. Mechanism of action The principal mechanism of action of the taxane class of drugs is the disruption of microtubule function. Microtubules are essential to cell division, and tax ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Doxorubicin
Doxorubicin, sold under the brand name Adriamycin among others, is a chemotherapy medication used to treat cancer. This includes breast cancer, bladder cancer, Kaposi's sarcoma, lymphoma, and acute lymphocytic leukemia. It is often used together with other chemotherapy agents. Doxorubicin is given by injection into a vein. Common side effects include hair loss, bone marrow suppression, vomiting, rash, and inflammation of the mouth. Other serious side effects may include allergic reactions such as anaphylaxis, heart damage, tissue damage at the site of injection, radiation recall, and treatment-related leukemia. People often experience red discoloration of the urine for a few days. Doxorubicin is in the anthracycline and antitumor antibiotic family of medications. It works in part by interfering with the function of DNA. Doxorubicin was approved for medical use in the United States in 1974. It is on the World Health Organization's List of Essential Medicines. Versions th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tricyclic Anti-depressants
Tricyclic antidepressants (TCAs) are a class of medications that are used primarily as antidepressants, which is important for the management of depression. They are second-line drugs next to SSRIs. TCAs were discovered in the early 1950s and were marketed later in the decade. They are named after their chemical structure, which contains three rings of atoms. Tetracyclic antidepressants (TeCAs), which contain four rings of atoms, are a closely related group of antidepressant compounds. Although TCAs are sometimes prescribed for depressive disorders, they have been largely replaced in clinical use in most parts of the world by newer antidepressants such as selective serotonin reuptake inhibitors (SSRIs), serotonin–norepinephrine reuptake inhibitors (SNRIs) and norepinephrine reuptake inhibitors (NRIs). Adverse effects have been found to be of a similar level between TCAs and SSRIs. Another class of antidepressants, tetracyclic antidepressants, are also used around the world, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
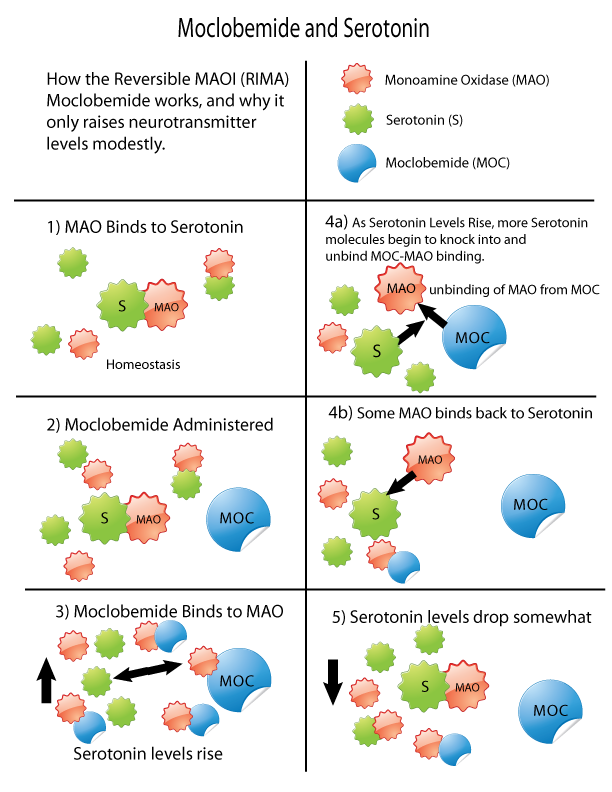
Monoamine Oxidase Inhibitors
Monoamine oxidase inhibitors (MAOIs) are a class of drugs that inhibit the activity of one or both monoamine oxidase enzymes: monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B). They are best known as effective antidepressants, especially for treatment-resistant depression and atypical depression. They are also used to treat panic disorder, social anxiety disorder, Parkinson's disease, and several other disorders. Reversible inhibitors of monoamine oxidase A (RIMAs) are a subclass of MAOIs that selectively and reversibly inhibit the MAO-A enzyme. RIMAs are used clinically in the treatment of depression and dysthymia. Due to their reversibility, they are safer in single-drug overdose than the older, irreversible MAOIs, and weaker in increasing the monoamines important in depressive disorder. RIMAs have not gained widespread market share in the United States. Medical uses MAOIs have been found to be effective in the treatment of panic disorder with agoraphobia, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selective Serotonin Reuptake Inhibitors
Selective serotonin reuptake inhibitors (SSRIs) are a class of drugs that are typically used as antidepressants in the treatment of major depressive disorder, anxiety disorders, and other psychological conditions. SSRIs increase the extracellular level of the neurotransmitter serotonin by limiting its reabsorption (reuptake) into the presynaptic cell. They have varying degrees of selectivity for the other monoamine transporters, with pure SSRIs having strong affinity for the serotonin transporter and only weak affinity for the norepinephrine and dopamine transporters. SSRIs are the most widely prescribed antidepressants in many countries. The efficacy of SSRIs in mild or moderate cases of depression has been disputed and may or may not be outweighed by side effects, especially in adolescent populations. Medical uses The main indication for SSRIs is major depressive disorder; however, they are frequently prescribed for anxiety disorders, such as social anxiety disorder, ge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NSAID
Non-steroidal anti-inflammatory drugs (NSAID) are members of a therapeutic drug class which reduces pain, decreases inflammation, decreases fever, and prevents blood clots. Side effects depend on the specific drug, its dose and duration of use, but largely include an increased risk of gastrointestinal ulcers and bleeds, heart attack, and kidney disease. The term ''non-steroidal'', common from around 1960, distinguishes these drugs from corticosteroids, which during the 1950s had acquired a bad reputation due to overuse and side-effect problems after their initial introduction in 1948. NSAIDs work by inhibiting the activity of cyclooxygenase enzymes (the COX-1 and COX-2 isoenzymes). In cells, these enzymes are involved in the synthesis of key biological mediators, namely prostaglandins, which are involved in inflammation, and thromboxanes, which are involved in blood clotting. There are two general types of NSAIDs available: non-selective, and COX-2 selective. Mos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutathione
Glutathione (GSH, ) is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, peroxides, lipid peroxides, and heavy metals. It is a tripeptide with a gamma peptide linkage between the carboxyl group of the glutamate side chain and cysteine. The carboxyl group of the cysteine residue is attached by normal peptide linkage to glycine. Biosynthesis and occurrence Glutathione biosynthesis involves two adenosine triphosphate-dependent steps: *First, γ-glutamylcysteine is synthesized from L- glutamate and cysteine. This conversion requires the enzyme glutamate–cysteine ligase (GCL, glutamate cysteine synthase). This reaction is the rate-limiting step in glutathione synthesis. *Second, glycine is added to the C-terminal of γ-glutamylcysteine. This condensation is catalyzed by glutathione synthetase. While all animal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
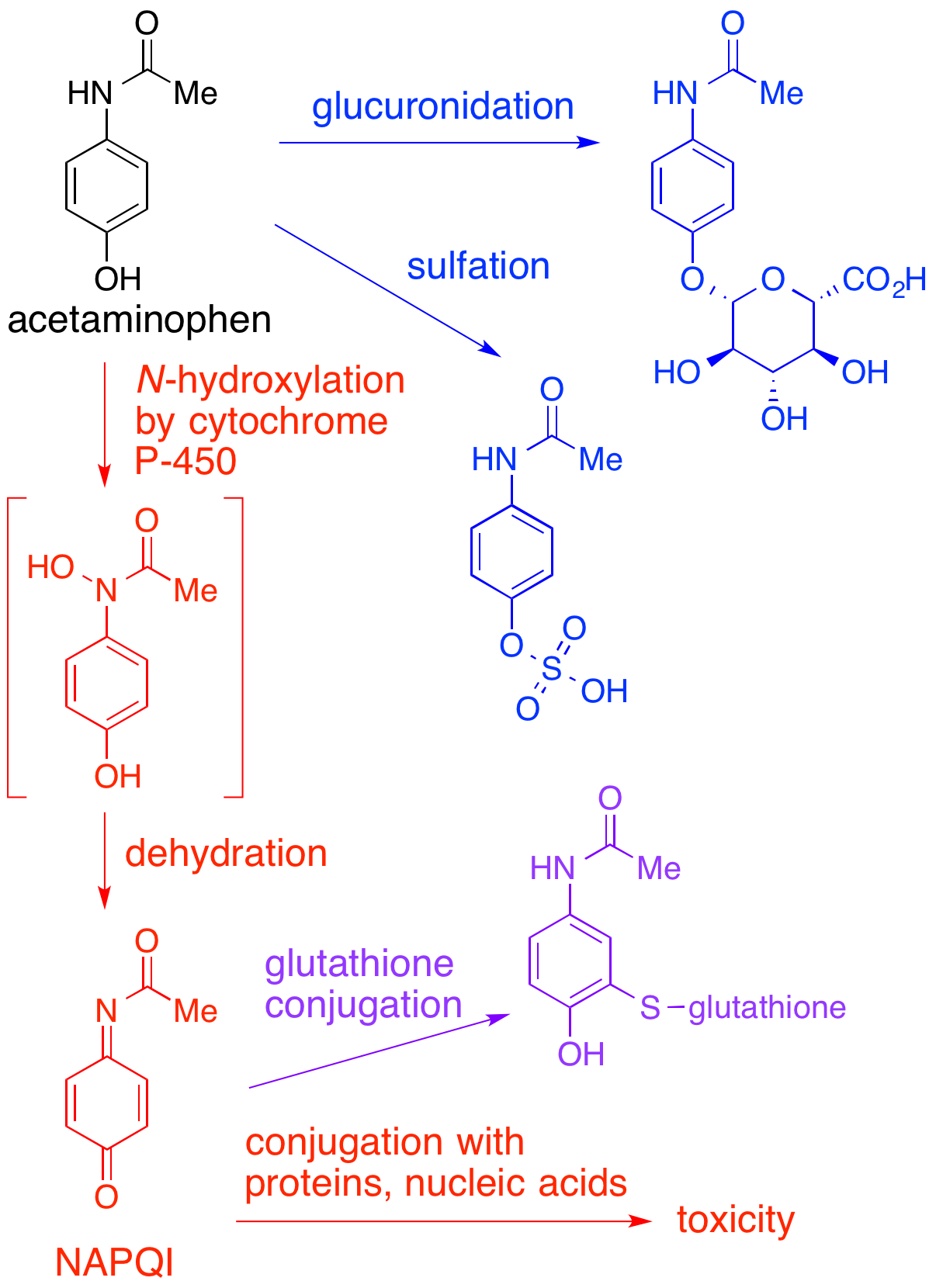
NAPQI
NAPQI, also known as NAPBQI or ''N''-acetyl-''p''-benzoquinone imine, is a toxic byproduct produced during the xenobiotic metabolism of the analgesic paracetamol (acetaminophen). It is normally produced only in small amounts, and then almost immediately detoxified in the liver. However, under some conditions in which NAPQI is not effectively detoxified (usually in the case of paracetamol overdose), it causes severe damage to the liver. This becomes apparent 3–4 days after ingestion and may result in death from fulminant liver failure several days after the overdose. Metabolism In adults, the primary metabolic pathway for paracetamol is glucuronidation. This yields a relatively non-toxic metabolite, which is excreted into bile and passed out of the body. A small amount of the drug is metabolized via the cytochrome P-450 pathway (to be specific, CYP3A4 and CYP2E1) into NAPQI, which is extremely toxic to liver tissue, as well as being a strong biochemical oxidizer. In an avera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |