|
Peroxide Value
Detection of peroxide gives the initial evidence of rancidity in unsaturated fats and oils. Other methods are available, but peroxide value is the most widely used. It gives a measure of the extent to which an oil sample has undergone primary oxidation, extent of secondary oxidation may be determined from p-anisidine test.Chemistry And Technology Of Oils And FatChemistry And Technology Of Oils And Fats By Dr. M.M. Chakrabarty The double bonds found in fats and oils play a role in autoxidation. Oils with a high degree of unsaturation are most susceptible to autoxidation. The best test for autoxidation (oxidative rancidity) is determination of the peroxide value. Peroxides are intermediates in the autoxidation reaction. Autoxidation is a free radical reaction involving oxygen that leads to deterioration of fats and oils which form off-flavours and off-odours. Peroxide value, concentration of peroxide in an oil or fat, is useful for assessing the extent to which spoilage has advanced ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxide
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable. The most common peroxide is hydrogen peroxide (), colloquially known simply as "peroxide". It is marketed as solutions in water at various concentrations. Many organic peroxides are known as well. In addition to hydrogen peroxide, some other major classes of peroxides are: * Peroxy acids, the peroxy derivatives of many familiar acids, examples being peroxymonosulfuric acid and peracetic acid, and their salts, one example of which is potassium peroxydisulfate. * Main group peroxides, compounds with the linkage (E = main group element). * Metal peroxides, examples being barium peroxide (), sodium peroxide () and zinc peroxide Zinc peroxide (ZnO2) appears as a bright yellow powder at room temperature. It was historically used as a surgical antiseptic. More recently zinc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements. Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important Reagent, chemical reagent and industrial chemical, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood Adhesive, glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the E number, food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is fundamental to all forms of life. When bound to coenzyme A, it is central to the metabolism of carbohydrates and fats. The global ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saponification Value
Saponification value or saponification number (SV or SN) represents the number of milligrams of potassium hydroxide (KOH) or sodium hydroxide (NaOH) required to saponify one gram of fat under the conditions specified. It is a measure of the average molecular weight (or chain length) of all the fatty acids present in the sample in form of triglycerides. The higher the saponification value, the lower the fatty acids average length, the lighter the mean molecular weight of triglycerides and vice versa. Practically, fats or oils with high saponification value (such as coconut and palm oil) are more suitable for soap making. Determination To determine saponification value, the sample is treated with an excess of alkali (usually an ethanolic solution of potassium hydroxide) for half an hour under reflux. The KOH is consumed by reaction with triglycerides, which consume two equivalents of base. Diglycerides consume two equivalents of KOH. Monoglycerides and free fatty acids, as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine Value
In chemistry, the iodine value (IV; also iodine adsorption value, iodine number or iodine index) is the mass of iodine in grams that is consumed by 100 grams of a chemical substance. Iodine numbers are often used to determine the degree of unsaturation in fats, oils and waxes. In fatty acids, unsaturation occurs mainly as double bonds which are very reactive towards halogens, the iodine in this case. Thus, the higher the iodine value, the more unsaturations are present in the fat. It can be seen from the table that coconut oil is very saturated, which means it is good for making soap. On the other hand, linseed oil is highly unsaturated, which makes it a drying oil, well suited for making oil paints. Principle The determination of iodine value is a particular example of iodometry. A solution of iodine is yellow/brown in color. When this is added to a solution to be tested, however, any chemical group (usually in this test double bonds) that react with iodine effecti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyl Value
In analytical chemistry, the hydroxyl value is defined as the number of milligrams of potassium hydroxide (KOH) required to neutralize the acetic acid taken up on acetylation of one gram of a chemical substance that contains free hydroxyl groups. The analytical method used to determine hydroxyl value traditionally involves acetylation of the free hydroxyl groups of the substance with acetic anhydride in pyridine solvent. After completion of the reaction, water is added, and the remaining unreacted acetic anhydride is converted to acetic acid and measured by titration with potassium hydroxide. The hydroxyl value can be calculated using the following equation. Note that a chemical substance may also have a measurable acid value affecting the measured endpoint of the titration. The acid value () of the substance, determined in a separate experiment, enters into this equation as a correction factor in the calculation of the hydroxyl value (): ::\mathrm = \frac + \mathrm Where i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epoxy Value
Epoxy is the family of basic components or Curing (chemistry), cured end products of epoxy resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide functional group is also collectively called ''epoxy''. The IUPAC name for an epoxide group is an oxirane. Epoxy resins may be reacted (cross-linked) either with themselves through catalytic homopolymerisation, or with a wide range of co-reactants including polyfunctional amines, acids (and acid anhydrides), phenols, alcohols and thiols (usually called mercaptans). These co-reactants are often referred to as hardeners or curatives, and the cross-linking reaction is commonly referred to as curing. Reaction of polyepoxides with themselves or with polyfunctional hardeners forms a thermosetting polymer, often with favorable mechanical properties and high thermal and chemical resistance. Epoxy has a wide range of applications, including metal coatings, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromine Number
In chemistry, the bromine number is the amount of bromine () in grams absorbed by of a sample. The number indicates the degree of unsaturation. The bromine number is useful as a measure of aliphatic unsaturation in gasoline samples. The California ARB, in its Motor Vehicle Fuels Compliance Assistance Program, indicated that every unit of bromine number is equivalent to twice the percentage point of olefin content in gasoline. So, a gasoline with bromine number 30 would have an olefin content of not more than 15 vol%. One refinery compiled a year's worth of data on bromine number and the corresponding olefin content of gasoline. The data showed that the bromine number of gasoline is about 2.4 times the olefin content. A gasoline with bromine number of 30 would then have an olefin content of about 12.5 percent by volume, which is slightly lower than ARB's assumption, but still is higher than the CaRFG maximum limit of 10 percent by volume. The bromine number is usually determine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine Value
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. Aromatic a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid Value
In chemistry, acid value (AV, acid number, neutralization number or acidity) is a number used to quantify the acidity of a given chemical substance. It is the quantity of base (usually potassium hydroxide (KOH)), expressed as milligrams of KOH required to neutralize the acidic constituents in 1 gram of a sample. The acid number is a measure of the number of carboxylic acid groups () in a chemical compound, such as a fatty acid, or in a mixture of compounds. In other words, it is a measure of free fatty acids (FFAs) present in a substance. In a typical procedure, a known amount of sample dissolved in an organic solvent (often isopropanol) and titrated with a solution of alcoholic potassium hydroxide (KOH) of known concentration using phenolphthalein as a colour indicator. The acid number for an oil sample is indicative of the age of the oil and can be used to determine when the oil must be changed. A liquid fat sample combined with neutralized 95% ethanol is titrated with s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Equivalence Point
The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been mixed. For an acid-base reaction the equivalence point is where the moles of acid and the moles of base would neutralize each other according to the chemical reaction. This does not necessarily imply a 1:1 molar ratio of acid:base, merely that the ratio is the same as in the chemical reaction. It can be found by means of an indicator, for example phenolphthalein or methyl orange. The endpoint (related to, but not the same as the equivalence point) refers to the point at which the indicator changes color in a colorimetric titration. Methods to determine the equivalence point Different methods to determine the equivalence point include: ;pH indicator: A pH indicator is a substance that changes color in response to a chemical change. An acid-base indicator (e.g., phenolphthalein) changes color depending on the pH. Redox indicators are a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox Titration
A redox titration is a type of titration based on a redox reaction between the analyte and titrant. It may involve the use of a redox indicator and/or a potentiometer. A common example of a redox titration is treating a solution of iodine with a reducing agent to produce iodide using a starch indicator to help detect the endpoint. Iodine (I2) can be reduced to iodide (I−) by, say, thiosulfate (, and when all iodine is spent the blue colour disappears. This is called an iodometric titration. Most often of all, the reduction of iodine to iodide is the last step in a series of reactions where the initial reactions are used to convert an unknown amount of the solute (the substance being analyzed) to an equivalent amount of iodine, which may then be titrated. Sometimes other halogens (or haloalkanes) than iodine are used in the intermediate reactions because they are available in better measurable standard solutions and/or react more readily with the solute. The extra steps in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
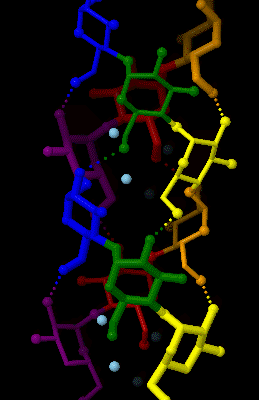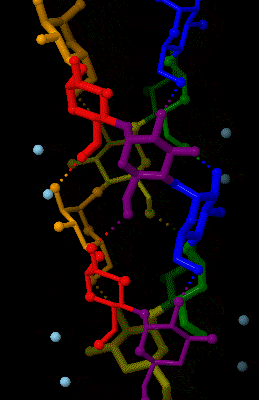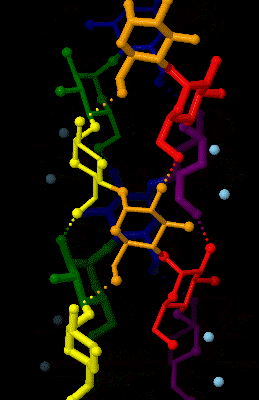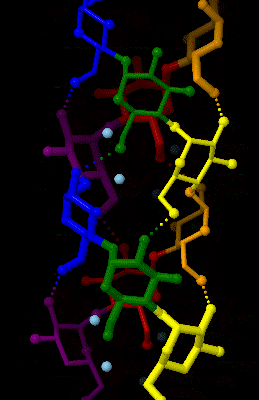
Amylose
Amylose is a polysaccharide made of α-D-glucose units, bonded to each other through α(1→4) glycosidic bonds. It is one of the two components of starch, making up approximately 20–30%. Because of its tightly packed helical structure, amylose is more resistant to digestion than other starch molecules and is therefore an important form of resistant starch. Structure Amylose is made up of α(1→4) bound glucose molecules. The carbon atoms on glucose are numbered, starting at the aldehyde (C=O) carbon, so, in amylose, the 1-carbon on one glucose molecule is linked to the 4-carbon on the next glucose molecule (α(1→4) bonds). The structural formula of amylose is pictured at right. The number of repeated glucose subunits (n) is usually in the range of 300 to 3000, but can be many thousands. There are three main forms of amylose chains can take. It can exist in a disordered amorphous conformation or two different helical forms. It can bind with itself in a double helix (A or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |