|
Pentapeptide Repeat
Pentapeptide repeats are a family of sequence motifs found in multiple tandem copies in protein molecules. Pentapeptide repeat proteins are found in all species, but they are found in many copies in cyanobacterial genomes. The repeats were first identified by Black and colleagues in the hglK protein. The later Bateman ''et al.'' showed that a large family of related pentapeptide repeat proteins existed. The function of these repeats is uncertain in most proteins. However, in the MfpA protein a DNA gyrase inhibitor it has been suggested that the pentapeptide repeat structure mimics the structure of DNA. The repeats form a regular right handed four sided beta helix structure known as the Rfr-fold. Sequence features The pentapeptide repeat is a feature seen in protein sequence. It can be approximately described using the 1-letter amino acid code as A(D/N)LXX, where X can be any amino acid . This repeating sequence can be seen in multiple sequence alignments and dot plots of pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sequence Motif
In biology, a sequence motif is a nucleotide or amino-acid sequence pattern that is widespread and usually assumed to be related to biological function of the macromolecule. For example, an ''N''-glycosylation site motif can be defined as ''Asn, followed by anything but Pro, followed by either Ser or Thr, followed by anything but Pro residue''. Overview When a sequence motif appears in the exon of a gene, it may encode the "structural motif" of a protein; that is a stereotypical element of the overall structure of the protein. Nevertheless, motifs need not be associated with a distinctive secondary structure. " Noncoding" sequences are not translated into proteins, and nucleic acids with such motifs need not deviate from the typical shape (e.g. the "B-form" DNA double helix). Outside of gene exons, there exist regulatory sequence motifs and motifs within the " junk", such as satellite DNA. Some of these are believed to affect the shape of nucleic acids (see for example RN ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Tandem Repeats
An array of protein tandem repeats is defined as several (at least two) adjacent copies having the same or similar sequence motifs. These periodic sequences are generated by internal duplications in both coding and non-coding genomic sequences. Repetitive units of protein tandem repeats are considerably diverse, ranging from the repetition of a single amino acid to domains of 100 or more residues. "Repeats" in proteins In proteins, a "repeat" is any sequence block that returns more than one time in the sequence, either in an identical or a highly similar form. The degree of similarity can be highly variable, with some repeats maintaining only a few conserved amino acid positions and a characteristic length. Highly degenerate repeats can be very difficult to detect from sequence alone. Structural similarity can help to identify repetitive patterns in sequence. Structure Repetitiveness does not in itself indicate anything about the structure of the protein. As a "rule of thu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Helix
A beta helix is a tandem protein repeat structure formed by the association of parallel beta strands in a helical pattern with either two or three faces. The beta helix is a type of solenoid protein domain. The structure is stabilized by inter-strand hydrogen bonds, protein-protein interactions, and sometimes bound metal ions. Both left- and right-handed beta helices have been identified. These structures are distinct from jelly-roll folds, a different protein structure sometimes known as a "double-stranded beta helix". The first beta-helix was observed in the enzyme pectate lyase, which contains a seven-turn helix that reaches 34 Å (3.4 nm) long. The P22 phage tail spike protein, a component of the P22 bacteriophage, has 13 turns and in its assembled homo trimer is 200 Å (20 nm) in length. Its interior is close-packed with no central pore and contains both hydrophobic residues and charged residues neutralized by salt bridges. Both pectate lyase and P22 tailspike prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PRP Align
PRP may refer to: Places * People's Republic of Poland, 1952-1990 * Pleasure Ridge Park, Louisville, in Kentucky, United States * Preston Park railway station, in Sussex, England Medicine and biology * Panretinal photocoagulation, a treatment for proliferative diabetic retinopathy * Penicillin-resistant pneumococci, a ''Streptococcus'' species resistant to antibiotics * Pityriasis rubra pilaris, a rare skin disorder * Platelet-rich plasma * Prion protein, a major constituent of the infectious prion * Progressive rubella panencephalitis, a viral neurological disorder * Proline rich proteins, a class of intrinsically unstructured proteins * Psychological refractory period, a period between processing multiple stimuli Mathematics and science * Probable prime, a number that satisfies some requirements for prime numbers * Parallel Redundancy Protocol, a network protocol providing fault tolerance * Pseudorandom permutation, a class of functions in cryptography * Petroleum Remediatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Sequence
Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthesis is most commonly performed by ribosomes in cells. Peptides can also be synthesized in the laboratory. Protein primary structures can be directly sequenced, or inferred from DNA sequences. Formation Biological Amino acids are polymerised via peptide bonds to form a long backbone, with the different amino acid side chains protruding along it. In biological systems, proteins are produced during translation by a cell's ribosomes. Some organisms can also make short peptides by non-ribosomal peptide synthesis, which often use amino acids other than the standard 20, and may be cyclised, modified and cross-linked. Chemical Peptides can be synthesised chemically via a range of laboratory methods. Chemical methods typically synthesise ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
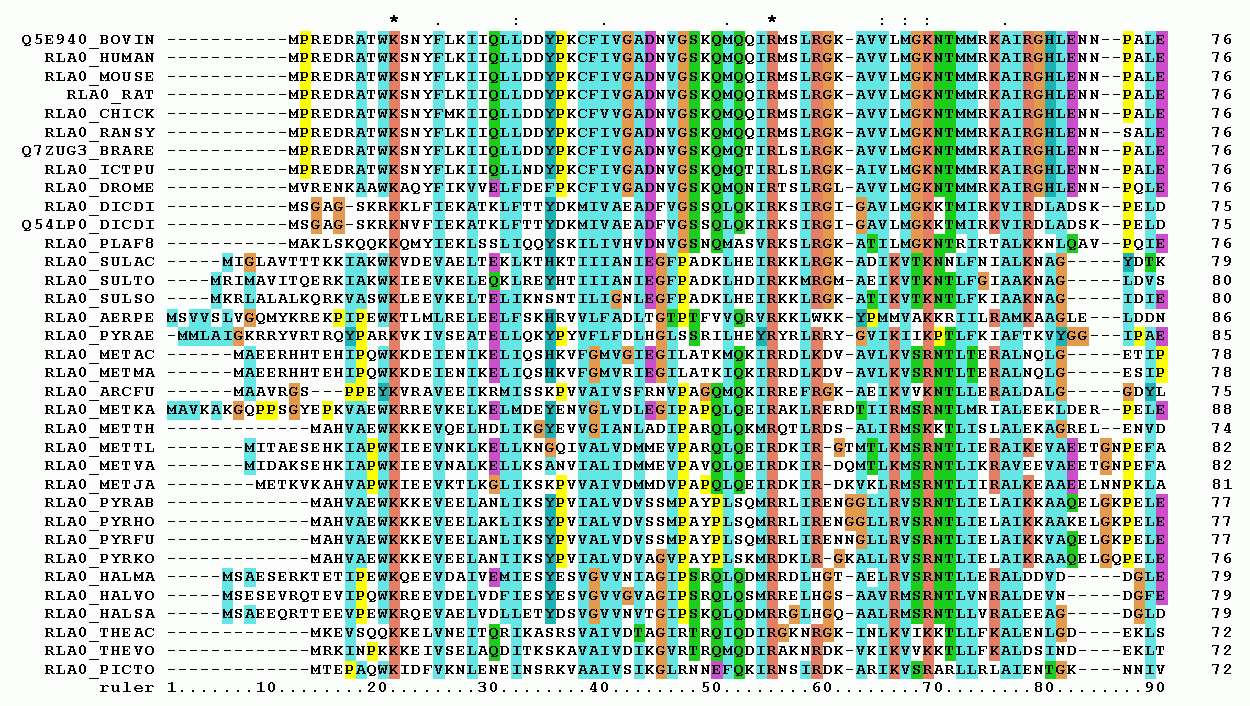
Multiple Sequence Alignment
Multiple sequence alignment (MSA) may refer to the process or the result of sequence alignment of three or more biological sequences, generally protein, DNA, or RNA. In many cases, the input set of query sequences are assumed to have an evolutionary relationship by which they share a linkage and are descended from a common ancestor. From the resulting MSA, sequence homology can be inferred and phylogenetic analysis can be conducted to assess the sequences' shared evolutionary origins. Visual depictions of the alignment as in the image at right illustrate mutation events such as point mutations (single amino acid or nucleotide changes) that appear as differing characters in a single alignment column, and insertion or deletion mutations (indels or gaps) that appear as hyphens in one or more of the sequences in the alignment. Multiple sequence alignment is often used to assess sequence conservation of protein domains, tertiary and secondary structures, and even individual amino acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dot Plot (bioinformatics)
In bioinformatics a dot plot is a graphical method for comparing two biological sequences and identifying regions of close similarity after sequence alignment. It is a type of recurrence plot. History One way to visualize the similarity between two protein or nucleic acid sequences is to use a similarity matrix, known as a dot plot. These were introduced by Gibbs and McIntyre in 1970 and are two-dimensional matrices that have the sequences of the proteins being compared along the vertical and horizontal axes. For a simple visual representation of the similarity between two sequences, individual cells in the matrix can be shaded black if residues are identical, so that matching sequence segments appear as runs of diagonal lines across the matrix. Interpretation Some idea of the similarity of the two sequences can be gleaned from the number and length of matching segments shown in the matrix. Identical proteins will obviously have a diagonal line in the center of the matrix. Inse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanothece
''Cyanothece'' is a genus of unicellular, diazotrophic, oxygenic photosynthesizing cyanobacteria. Modern organisms and cellular organization In 1976, Jiří Komárek defined the prokaryotic cyanobacteria genus ''Cyanothece'' as distinct from ''Synechococcus'' NAG 1949. Organisms in both genera share characteristics in addition to being oxygenic phototrophs. They are both unicellular, forming aggregates, but not found in mucilaginous colonies. They may have a thin mucilage layer around each cell. Both genera also divide by binary fission along an axis perpendicular to the cell's longitudinal axis. A handful of characteristics distinguish the two genera. While ''Synechococcus'' species are usually cylindrical, ''Cyanothece'' species are normally oval and longer than 3 μm., ''Cyanothece’s'' outer cell wall layer is relatively thick and contains spherical, glassy Vesicle (biology and chemistry), vesicles whose function has yet to be defined. ''Cyanothece’s'' nucleoids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |