|
Pentagestrone Acetate
Pentagestrone acetate (PGA), sold under the brand names Gestovis and Gestovister, is a progestin which was described in the literature in 1960 and was introduced by Vister in Italy in 1961. It is the 3-cyclopentyl enol ether of 17α-hydroxyprogesterone acetate. PGA, along with quingestrone (the 3-cyclopentyl enol ether of progesterone), is said to have very similar properties to those of dydrogesterone, a pure progestogen and close analogue of progesterone. PGA is orally active, was provided in 10 and 20 mg capsules, and has been used to treat habitual abortion and menstrual disorders at a dosage of 10 to 20 mg/day. It has been said to have equivalent potency to intramuscular progesterone. The combination of 20 mg/day PGA and 100 μg/day mestranol is an effective ovulation inhibitor in women. The effective dosage of PGA in the menstrual delay test has been studied. Chemistry PGA, also known as 17α-acetoxyprogesterone 3-cyclopentyl enol ether, is a syn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oral Administration
Oral administration is a route of administration where a substance is taken through the mouth. Per os abbreviated to P.O. is sometimes used as a direction for medication to be taken orally. Many medications are taken orally because they are intended to have a systemic effect, reaching different parts of the body via the bloodstream, for example. Oral administration can be easier and less painful than other routes, such as injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients willing and able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
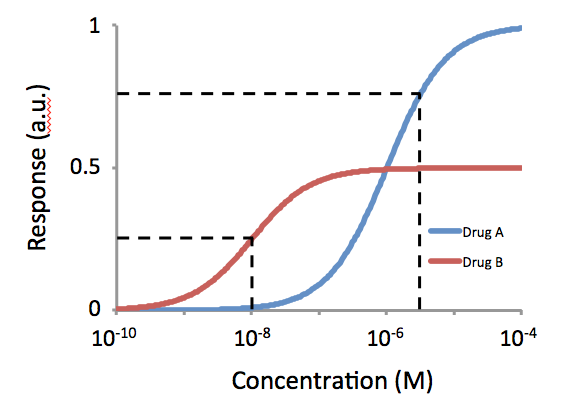
Potency (pharmacology)
In the field of pharmacology, potency is a measure of drug activity expressed in terms of the amount required to produce an effect of given intensity. A highly potent drug (e.g., fentanyl, alprazolam, risperidone, bumetanide, bisoprolol) evokes a given response at low concentrations, while a drug of lower potency (meperidine, diazepam, ziprasidone, furosemide, metoprolol) evokes the same response only at higher concentrations. Higher potency does not necessarily mean greater effectiveness or more side effects. The IUPHAR The International Union of Basic and Clinical Pharmacology (IUPHAR) is a voluntary, non-profit association representing the interests of scientists in pharmacology-related fields to facilitate ''Better Medicines through Global Education and Resear ... has stated that 'potency' is ''"an imprecise term that should always be further defined"'', for instance as EC_, IC_, ED_, LD_ and so on. See also * Reaction inhibitor § Potency References Further readin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quingestrone
Quingestrone, also known as progesterone 3-cyclopentyl enol ether (PCPE) and sold under the brand name Enol-Luteovis, is a progestin medication which was previously used in birth control pills in Italy but is now no longer marketed. It is taken by mouth. Quingestrone is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone. It has weak glucocorticoid activity. Quingestrone was introduced for medical use by 1962. It is no longer available. Medical uses Quingestrone was formerly used in combination with ethinylestradiol or mestranol in combined birth control pills in Italy. The medication was studied in the clinical prevention of miscarriage during pregnancy, but insufficient efficacy was observed at the dosage assessed (100 mg/day orally). Side effects Pharmacology Pharmacodynamics Along with the retroprogesterone derivative dydrogesterone, quingestrone has been ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Progesterone 3-acetyl Enol Ether
Progesterone 3-acetyl enol ether, also known as progesterone acetate, as well as 3-acetoxypregna-3,5-dien-20-one, is a progestin which was never marketed.Rao, P. N., & Edwards, B. E. (1967). ''U.S. Patent No. 3,321,495.'' Washington, DC: U.S. Patent and Trademark Office. It was reported to possess similar potency to progesterone and hydroxyprogesterone caproate in the rabbit endometrial carbonic anhydrase test, a bioassay of progestogenic activity. In addition, it was able to maintain pregnancy in animals. Progesterone 3-acetyl enol ether is closely related to quingestrone, which is also known as progesterone 3-cyclopentyl enol ether and was formerly marketed as an oral contraceptive. The 3-acetyl ether may be cleaved from progesterone 3-acetyl enol ether ''in vivo'' and, based on its chemical structure, this may result in the transformation of progesterone 3-acetyl enol ether into 3α-dihydroprogesterone and/or 3β-dihydroprogesterone. 3β-Dihydroprogesterone has been rep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentagestrone
Pentagestrone (), also known as 17α-hydroxyprogesterone 3-cyclopentyl enol ether, is a steroidal progestin of the 17α-hydroxyprogesterone group that was never marketed. An acetate ester, pentagestrone acetate Pentagestrone acetate (PGA), sold under the brand names Gestovis and Gestovister, is a progestin which was described in the literature in 1960 and was introduced by Vister in Italy in 1961. It is the 3- cyclopentyl enol ether of 17α-hydroxypr ... (Gestovis, Gestovister), has been marketed for clinical use. Pentagestrone was described in the literature in 1960. See also * Quingestrone References Abandoned drugs Cyclopentyl ethers Pregnanes Progestogen ethers Progestogens {{steroid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
17α-hydroxyprogesterone
17α-Hydroxyprogesterone (17α-OHP), also known as 17-OH progesterone (17-OHP), or hydroxyprogesterone (OHP), is an endogenous progestogen steroid hormone related to progesterone. It is also a chemical intermediate in the biosynthesis of many other endogenous steroids, including androgens, estrogens, glucocorticoids, and mineralocorticoids, as well as neurosteroids. Biological activity 17α-OHP is an agonist of the progesterone receptor (PR) similarly to progesterone, albeit weakly in comparison. In addition, it is an antagonist of the mineralocorticoid receptor (MR) as well as a partial agonist of the glucocorticoid receptor (GR), albeit with very low potency ( EC50 >100-fold less relative to cortisol) at the latter site, also similarly to progesterone. Biochemistry Biosynthesis 17α-OHP is derived from progesterone via 17α-hydroxylase (encoded by CYP17A1) 17α-OHP increases in the third trimester of pregnancy primarily due to fetal adrenal production. This steroid is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Progesterone (medication)
Progesterone (P4) is a medication and naturally occurring steroid hormone. It is a progestogen and is used in combination with estrogens mainly in hormone therapy for menopausal symptoms and low sex hormone levels in women. It is also used in women to support pregnancy and fertility and to treat gynecological disorders. Progesterone can be taken by mouth, in through the vagina, and by injection into muscle or fat, among other routes. A progesterone vaginal ring and progesterone intrauterine device used for birth control also exist in some areas of the world. Progesterone is well tolerated and often produces few or no side effects. However, a number of side effects are possible, for instance mood changes. If progesterone is taken by mouth or at high doses, certain central side effects including sedation, sleepiness, and cognitive impairment can also occur. The medication is a naturally occurring progestogen and hence is an agonist of the progesterone receptor (PR), t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Derivative
In chemistry, a derivative is a compound that is derived from a similar compound by a chemical reaction. In the past, derivative also meant a compound that ''can be imagined to'' arise from another compound, if one atom or group of atoms is replaced with another atom or group of atoms, but modern chemical language now uses the term structural analog for this meaning, thus eliminating ambiguity. The term "structural analogue" is common in organic chemistry. In biochemistry, the word is used for compounds that at least theoretically can be formed from the precursor compound. Chemical derivatives may be used to facilitate analysis. For example, melting point (MP) analysis can assist in identification of many organic compounds. A crystalline derivative may be prepared, such as a semicarbazone or 2,4-dinitrophenylhydrazone (derived from aldehydes or ketones), as a simple way of verifying the identity of the original compound, assuming that a table of derivative MP values is available ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steroid
A steroid is a biologically active organic compound with four rings arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as signaling molecules. Hundreds of steroids are found in plants, animals and fungi. All steroids are manufactured in cells from the sterols lanosterol (opisthokonts) or cycloartenol (plants). Lanosterol and cycloartenol are derived from the cyclization of the triterpene squalene. The steroid core structure is typically composed of seventeen carbon atoms, bonded in four " fused" rings: three six-member cyclohexane rings (rings A, B and C in the first illustration) and one five-member cyclopentane ring (the D ring). Steroids vary by the functional groups attached to this four-ring core and by the oxidation state of the rings. Sterols are forms of steroids with a hydroxy group at position three and a skeleton derived from cholestane. ''A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pregnane
Pregnane, also known as 17β-ethylandrostane or as 10β,13β-dimethyl-17β-ethylgonane, is a C21 steroid and, indirectly, a parent of progesterone. It is a parent hydrocarbon for two series of steroids stemming from 5α-pregnane (originally allopregnane) and 5β-pregnane (17β-ethyletiocholane). It has a gonane core. 5β-Pregnane is the parent of pregnanediones, pregnanolones, and pregnanediols, and is found largely in urine as a metabolic product of 5β-pregnane compounds. Pregnanes Pregnanes are steroid derivatives with carbons present at positions 1 through 21. Most biologically significant pregnane derivatives fall into one of two groups: pregnenes and pregnadienes. Another class is pregnatrienes. Pregnenes Pregnenes have a double bond. Examples include: * Cortisone * Hydrocortisone * Progesterone Pregnadienes Pregnadienes have two double bonds. Examples include: * Cyproterone acetate * Danazol * Fluocinonide See also * 5β-Pregnane * Pregnanedione * Pregna ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Menstrual Delay Test
Menstruation (also known as a period, among other colloquial terms) is the regular discharge of blood and mucosal tissue from the inner lining of the uterus through the vagina. The menstrual cycle is characterized by the rise and fall of hormones. Menstruation is triggered by falling progesterone levels and is a sign that pregnancy has not occurred. The first period, a point in time known as menarche, usually begins between the ages of 12 and 15. Menstruation starting as young as 8 years would still be considered normal. The average age of the first period is generally later in the developing world, and earlier in the developed world. The typical length of time between the first day of one period and the first day of the next is 21 to 45 days in young women. In adults, the range is between 21 and 31 days with the average being 28 days. Bleeding usually lasts around 2 to 7 days. Periods stop during pregnancy and typically do not resume during the initial months of breastfe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |