|
Parallel Study
A parallel study is a type of clinical study where two groups of treatments, A and B, are given so that one group receives only A while another group receives only B. Other names for this type of study include "between patient" and "non-crossover". This is unlike a crossover study where at first one group receives treatment A and later followed by treatment B while the other group receives treatment B followed by treatment A. There are, however, certain characteristics that allow for differentiation between these two types of trials. For example, a parallel study would be more appropriate if any concerns about carryover effects were present. This type of study might also be more beneficial if the disease or disorder being studied has a likely chance of progression during the time in which the study takes place. One significant issue with parallel studies, though, is the concept of intra subject variability, which is defined as variability in response occurring within the same patie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Study
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small pilot studies, and subsequently conduct progressively larger scale comparative studies. Clinical trials can vary in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medical Treatment
A therapy or medical treatment (often abbreviated tx, Tx, or Tx) is the attempted remediation of a health problem, usually following a medical diagnosis. As a rule, each therapy has indications and contraindications. There are many different types of therapy. Not all therapies are effective. Many therapies can produce unwanted adverse effects. ''Medical treatment'' and ''therapy'' are generally considered synonyms. However, in the context of mental health, the term ''therapy'' may refer specifically to psychotherapy. History Before the creating of therapy as a formal procedure, people told stories to one another to inform and assist about the world. The term "healing through words" was used over 3,500 years ago in Greek and Egyptian writing. The term psychotherapy was invented in the 19th century, and psychoanalysis was founded by Sigmund Freud under a decade later. Semantic field The words ''care'', ''therapy'', ''treatment'', and ''intervention'' overlap in a sem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crossover Study
In medicine, a crossover study or crossover trial is a longitudinal study in which subjects receive a sequence of different treatments (or exposures). While crossover studies can be observational studies, many important crossover studies are controlled experiments, which are discussed in this article. Crossover designs are common for experiments in many scientific disciplines, for example psychology, pharmaceutical science, and medicine. Randomized, controlled crossover experiments are especially important in health care. In a randomized clinical trial, the subjects are randomly assigned to different arms of the study which receive different treatments. When the trial has a repeated measures design, the same measures are collected multiple times for each subject. A crossover trial has a repeated measures design in which each patient is assigned to a sequence of two or more treatments, of which one may be a standard treatment or a placebo. Nearly all crossover are designed to ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
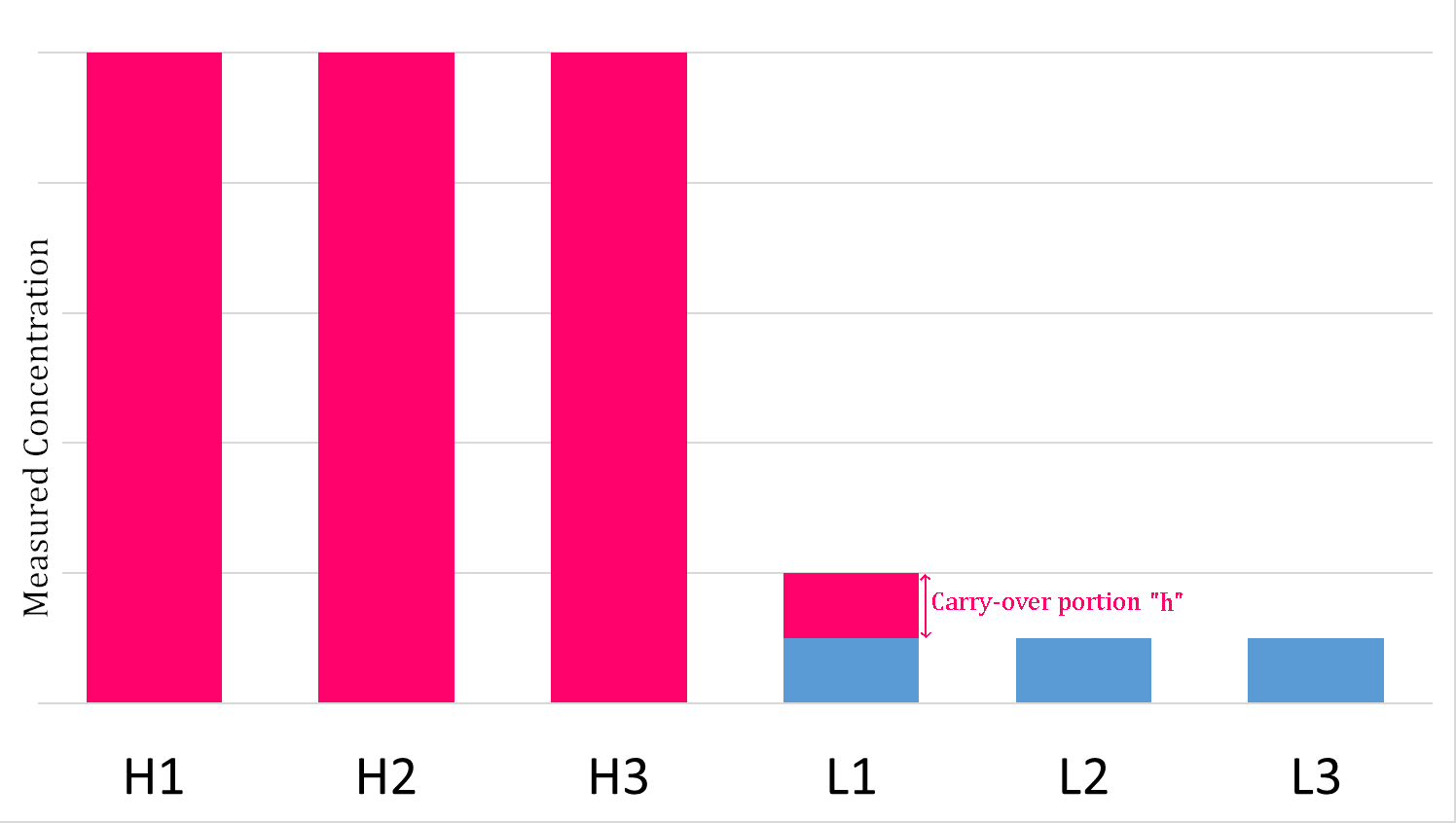
Carryover Effect
The carryover effect is a term used in clinical chemistry to describe the transfer of unwanted material from one container or mixture to another. It describes the influence of one sample upon the following one. It may be from a specimen, or a reagent, or even the washing medium. The significance of carry over is that even a small amount can lead to erroneous results. Carryover effect in clinical laboratory Carryover experiments are widely used for clinical chemistry and immunochemistry analyzers to evaluate and validate carryover effects. The pipetting and washing systems in an automated analyzer are designed to continuously cycle between the aspiration of patient specimens and cleaning. An obvious concern is a potential for carryover of analyte from one patient specimen into one or more following patient specimens, which can falsely increase or decrease the measured analyte concentration. Specimen carryover is typically addressed by judicious choice of probe material, probe de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intra Subject Variability
''Intra'' may refer to: * ''Intra'' (album), a 2005 album by metal band Ram-Zet * Intra, a city now part of Verbania, Italy * Intra Airways, British airline * Intra (software), anti Internet censorship Android app by Google. * Intra-frame coding, a video compression technique that utilizes I-frames, a video compression picture type * Intra Bank, Lebanese bank * Intra (Teka), Norwegian company * Groupe INTRA, French emergency response organization * AVC-Intra, a type of video coding People with the surname *Enrico Intra Enrico Intra (born 3 July 1935 in Milan, Italy) is an Italian jazz pianist, composer, Conductor (music), conductor. During his long career, Intra has collaborated with such notable musicians as Gerry Mulligan, Franco Cerri, Lee Konitz, Milt Jackso ... (born 1935), Italian pianist, composer, and conductor * Giovanni Intra (1968–2002), New Zealand artist, writer, and art dealer See also * {{disambiguation, surname ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Randomization
Randomization is the process of making something random. Randomization is not haphazard; instead, a random process is a sequence of random variables describing a process whose outcomes do not follow a deterministic pattern, but follow an evolution described by probability distributions. For example, a random sample of individuals from a population refers to a sample where every individual has a known probability of being sampled. This would be contrasted with nonprobability sampling where arbitrary individuals are selected. In various contexts, randomization may involve: * generating a random permutation of a sequence (such as when shuffling cards); * selecting a random sample of a population (important in statistical sampling); * allocating experimental units via random assignment to a treatment or control condition; * generating random numbers (random number generation); or * transforming a data stream (such as when using a scrambler in telecommunications). Applications Randomi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Placebo
A placebo ( ) is a substance or treatment which is designed to have no therapeutic value. Common placebos include inert tablets (like sugar pills), inert injections (like Saline (medicine), saline), sham surgery, and other procedures. In general, placebos can affect how patients perceive their condition and encourage the body's chemical processes for relieving pain and a few other symptoms, but have no impact on the disease itself. Improvements that patients experience after being treated with a placebo can also be due to unrelated factors, such as regression to the mean (a statistical effect where an unusually high or low measurement is likely to be followed by a less extreme one). The use of placebos in clinical medicine raises ethical concerns, especially if they are disguised as an active treatment, as this introduces dishonesty into the doctor–patient relationship and bypasses informed consent. While it was once assumed that this deception was necessary for placebos to have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trials
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small pilot studies, and subsequently conduct progressively larger scale comparative studies. Clinical trials can vary i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)