|
PLEKHA7
PLEKHA7 (Pleckstrin homology domain-containing family A member 7) is an adherens junction (AJ) protein, involved in the junction's integrity and stability. History The protein was discovered in Masatoshi Takeichi’s lab while looking for potential binding partners for the N-terminal region of p120. PLEKHA7 was identified by mass spectrometry in lysates of human intestinal carcinoma (Caco-2) cells in a GST-pull down using N-terminal GST-fusion p120 catenin as bait. It was also independently discovered in Sandra Citi’s group as a protein interacting with globular head domain of the Paracingulin in a yeast two-hybrid screen. PLEKHA7 localizes at epithelial zonular AJs. Structure The structure of PLEKHA7 is characterized by two WW domains followed by a Pleckstrin homology domain (PH) in the N-terminal region. In the C-terminal half, the protein contains three coiled coil (CC) domains and two Proline-rich (Pro) domains. PLEKHA7 has been detected in different isoforms in a t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paracingulin
Cingulin-like protein 1, also known as paracingulin or junction-associated-coiled-coil protein (JACOP), is a protein which is encoded by the ''CGNL1'' gene. The paracingulin polypeptide comprises a globular N-terminal "head" domain and an α-helical C-terminal domain which is presumed to form a coiled-coil dimer. Paracingulin is a paralog of cingulin that arose probably from gene duplication. The CGNL1 gene is conserved among different vertebrate species and has not been so far identified in invertebrates. The homology search highlights that paracingulin and cingulin have 39% identity in the rod tail sequences. They possess also two highly homologous regions in their N-terminus “head” domain including the ZIM region (ZO-1 interaction motif). The etymology of the name cingulin comes from the Latin "cingere" which means « to form a belt around ». Both, cingulin and paracingulin are localized in the cytoplasmic face of tight junctions (TJ). The prefix « para » refers to “pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
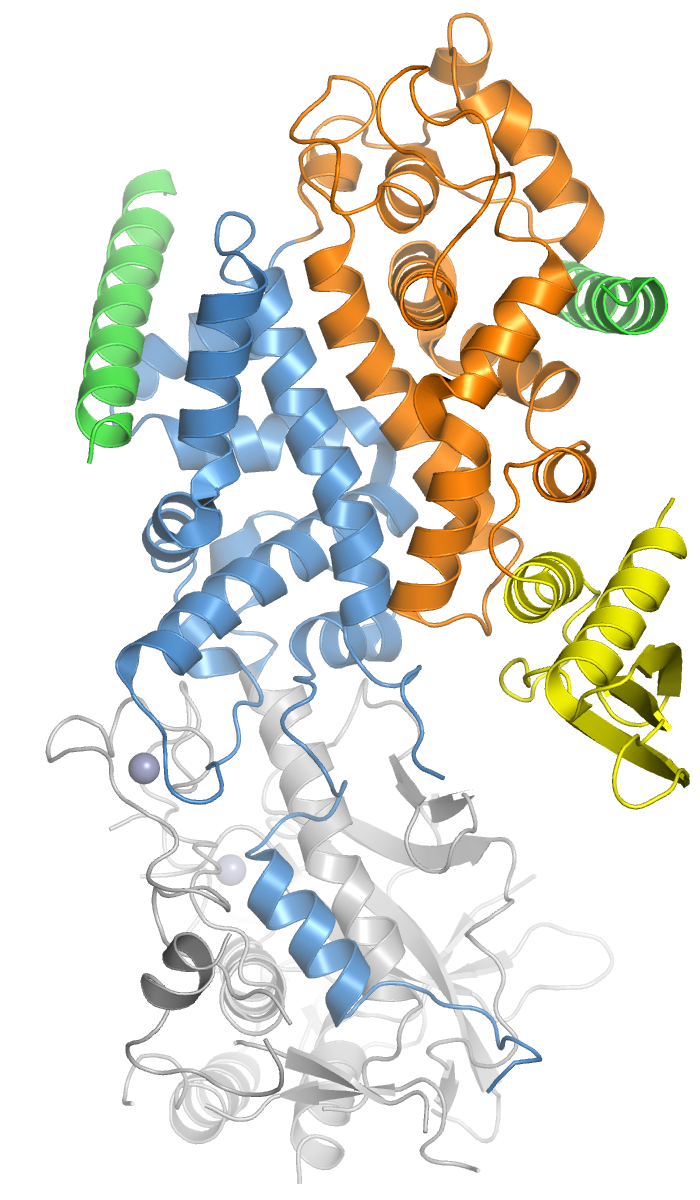
Pleckstrin Homology Domain
Pleckstrin homology domain (PH domain) or (PHIP) is a protein domain of approximately 120 amino acids that occurs in a wide range of proteins involved in intracellular signaling or as constituents of the cytoskeleton. This domain can bind phosphatidylinositol lipids within biological membranes (such as phosphatidylinositol (3,4,5)-trisphosphate and phosphatidylinositol (4,5)-bisphosphate), and proteins such as the βγ-subunits of heterotrimeric G proteins, and protein kinase C. Through these interactions, PH domains play a role in recruiting proteins to different membranes, thus targeting them to appropriate cellular compartments or enabling them to interact with other components of the signal transduction pathways. Lipid binding specificity Individual PH domains possess specificities for phosphoinositides phosphorylated at different sites within the inositol ring, e.g., some bind phosphatidylinositol (4,5)-bisphosphate but not phosphatidylinositol (3,4,5)-trisphosphate or p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microtubules
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement. Microtubules play an important role in a number of cellular processes. They are involved in maintaining the structure of the cell and, together with microfilaments and intermediate filaments, they form the cytoskeleton. They also make up the internal structure of cilia and flagella. They provide platforms for intracellular transport and are involved in a variety of cellular processes, including the movement of secretory vesicles, organell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adherens Junction
Adherens junctions (or zonula adherens, intermediate junction, or "belt desmosome") are protein complexes that occur at cell–cell junctions, cell–matrix junctions in epithelial and endothelial tissues, usually more basal than tight junctions. An adherens junction is defined as a cell junction whose cytoplasmic face is linked to the actin cytoskeleton. They can appear as bands encircling the cell (zonula adherens) or as spots of attachment to the extracellular matrix (focal adhesion). Adherens junctions uniquely disassemble in uterine epithelial cells to allow the blastocyst to penetrate between epithelial cells. A similar cell junction in non-epithelial, non-endothelial cells is the fascia adherens. It is structurally the same, but appears in ribbonlike patterns that do not completely encircle the cells. One example is in cardiomyocytes. Proteins Adherens junctions are composed of the following proteins: * cadherins. The cadherins are a family of transmembrane proteins tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proto-oncogene Tyrosine-protein Kinase Src
Proto-oncogene tyrosine-protein kinase Src, also known as proto-oncogene c-Src, or simply c-Src (cellular Src; pronounced "sarc", as it is short for sarcoma), is a non-receptor tyrosine kinase protein that in humans is encoded by the ''SRC'' gene. It belongs to a family of Src family kinases and is similar to the v-Src (viral Src) gene of Rous sarcoma virus. It includes an SH2 domain, an SH3 domain and a tyrosine kinase domain. Two transcript variants encoding the same protein have been found for this gene. c-Src phosphorylates specific tyrosine residues in other tyrosine kinases. It plays a role in the regulation of embryonic development and cell growth. An elevated level of activity of c-Src is suggested to be linked to cancer progression by promoting other signals. Mutations in c-Src could be involved in the malignant progression of colon cancer. c-Src should not be confused with CSK (C-terminal Src kinase), an enzyme that phosphorylates c-Src at its C-terminus and provides n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorylated
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. Protein phosphorylation often activates (or deactivates) many enzymes. Glucose Phosphorylation of sugars is often the first stage in their catabolism. Phosphorylation allows cells to accumulate sugars because the phosphate group prevents the molecules from diffusing back across their transporter. Phosphorylation of glucose is a key reaction in sugar metabolism. The chemical equation for the conversion of D-glucose to D-glucose-6-phosphate in the first step of glycolysis is given by :D-glucose + ATP → D-glucose-6-phosphate + ADP : ΔG° = −16.7 kJ/mol (° indicates measurement at standard condition) Hepatic cells are freely permeable to glucose, and the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Greek ''tyrós'', meaning ''cheese'', as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a Hydrophobe, hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is Genetic code, encoded by the Genetic code#Codons, codons UAC and UAU in messenger RNA. Functions Aside from being a proteinogenic amino acid, tyrosine has a special role by virtue of the phenol functionality. It occurs in proteins that are part of signal transduction processes and functions as a receiver of phosphate groups that are transferred by way of protein kinases. Phosphorylation of the hyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epithelial Cells
Epithelium or epithelial tissue is one of the four basic types of animal tissue, along with connective tissue, muscle tissue and nervous tissue. It is a thin, continuous, protective layer of compactly packed cells with a little intercellular matrix. Epithelial tissues line the outer surfaces of organs and blood vessels throughout the body, as well as the inner surfaces of cavities in many internal organs. An example is the epidermis, the outermost layer of the skin. There are three principal shapes of epithelial cell: squamous (scaly), columnar, and cuboidal. These can be arranged in a singular layer of cells as simple epithelium, either squamous, columnar, or cuboidal, or in layers of two or more cells deep as stratified (layered), or ''compound'', either squamous, columnar or cuboidal. In some tissues, a layer of columnar cells may appear to be stratified due to the placement of the nuclei. This sort of tissue is called pseudostratified. All glands are made up of epithelia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DGCR8
The microprocessor complex subunit DGCR8 ''(DiGeorge syndrome critical region 8)'' is a protein that in humans is encoded by the gene. In other animals, particularly the common model organisms ''Drosophila melanogaster'' and ''Caenorhabditis elegans'', the protein is known as ''Pasha'' (partner of Drosha). It is a required component of the RNA interference pathway. Function The subunit DGCR8 is localized to the cell nucleus and is required for microRNA (miRNA) processing. It binds to the other subunit Drosha, an RNase III enzyme, to form the microprocessor complex that cleaves a primary transcript known as pri-miRNA to a characteristic stem-loop structure known as a pre-miRNA, which is then further processed to miRNA fragments by the enzyme Dicer. DGCR8 contains an RNA-binding domain and is thought to bind pri-miRNA to stabilize it for processing by Drosha. DGCR8 is also required for some types of DNA repair. Removal of UV-induced DNA Pyrimidine dimer, photoproducts, during ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drosha
Drosha is a Class 2 ribonuclease III enzyme that in humans is encoded by the ''DROSHA'' (formerly ''RNASEN'') gene. It is the primary nuclease that executes the initiation step of miRNA processing in the nucleus. It works closely with DGCR8 and in correlation with Dicer. It has been found significant in clinical knowledge for cancer prognosisSlack FJ, Weidhaas JB (December 2008). "MicroRNA in cancer prognosis". ''The New England Journal of Medicine.'' 359 (25): 2720-2. and HIV-1 replication.Swaminathan, G., Navas-Martín, S., & Martín-García, J. (2014). MicroRNAs and HIV-1 infection: antiviral activities and beyond. ''Journal of molecular biology'', ''426''(6), 1178-1197. History Human Drosha was cloned in 2000 when it was identified as a nuclear dsRNA ribonuclease involved in the processing of ribosomal RNA precursors. The other two human enzymes that participate in the processing and activity of miRNA are the Dicer and Argonaute proteins. Recently, proteins like Drosha h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microprocessor Complex
The microprocessor complex is a protein complex involved in the early stages of processing microRNA (miRNA) and RNA interference (RNAi) in animal cells. The complex is minimally composed of the ribonuclease enzyme Drosha and the dimeric RNA-binding protein DGCR8 (also known as Pasha in non-human animals), and cleaves primary miRNA substrates to pre-miRNA in the cell nucleus. Microprocessor is also the smaller of the two multi-protein complexes that contain human Drosha. Composition The microprocessor complex consists minimally of two proteins: Drosha, a ribonuclease III enzyme; and DGCR8, a double-stranded RNA binding protein. (DGCR8 is the name used in mammalian genetics, abbreviated from "DiGeorge syndrome critical region 8"; the homologous protein in model organisms such as flies and worms is called ''Pasha'', for ''Pa''rtner of Dro''sha''.) The stoichiometry of the minimal complex was at one point experimentally difficult to determine, but it has been demonstrated to be a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphatidylinositol 4-kinase
In enzymology, a 1-phosphatidylinositol 4-kinase () is an enzyme that catalyzes the chemical reaction :ATP + 1-phosphatidyl-1D-myo-inositol \rightleftharpoons ADP + 1-phosphatidyl-1D-myo-inositol 4-phosphate Thus, the two substrates of this enzyme are ATP and 1-phosphatidyl-1D-myo-inositol, whereas its two products are ADP and 1-phosphatidyl-1D-myo-inositol 4-phosphate. This enzyme belongs to the family of transferases, specifically those transferring phosphorus-containing groups (phosphotransferases) with an alcohol group as acceptor. The systematic name of this enzyme class is ATP:1-phosphatidyl-1D-myo-inositol 4-phosphotransferase. Other names in common use include phosphatidylinositol kinase (phosphorylating), phosphatidylinositol 4-kinase, phosphatidylinositol kinase, type II phosphatidylinositol kinase, PI kinase, and PI 4-kinase. This enzyme participates in inositol phosphate metabolism and phosphatidylinositol signaling system. Structural studies As of late 2007, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |