|
PHLPP
The PHLPP isoforms (PH domain and Leucine rich repeat Protein Phosphatases) are a pair of protein phosphatases, PHLPP1 and PHLPP2, that are important regulators of Akt serine-threonine kinases ( Akt1, Akt2, Akt3) and conventional/novel protein kinase C (PKC) isoforms. PHLPP may act as a tumor suppressor in several types of cancer due to its ability to block growth factor-induced signaling in cancer cells. PHLPP dephosphorylates Ser-473 (the hydrophobic motif) in Akt, thus partially inactivating the kinase. In addition, PHLPP dephosphorylates conventional and novel members of the protein kinase C family at their hydrophobic motifs, corresponding to Ser-660 in PKCβII. Domain structure PHLPP is a member of the PPM family of phosphatases, which requires magnesium or manganese for their activity and are insensitive to most common phosphatase inhibitors, including kadaic acid PHLPP1 and PHLPP2 have a similar domain structure, which includes a putative Ras association do ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PHLPP (gene)
PH domain and leucine rich repeat protein phosphatase, also known as PHLPP, is an enzyme which in humans is encoded by the ''PHLPP'' gene In biology, the word gene (from , ; "... Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a b .... See also * PHLPP References Further reading * * * * * * * * * * {{gene-18-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PHLPPL
PH domain and leucine rich repeat protein phosphatase-like, also known as PHLPPL, is an enzyme which in humans is encoded by the ''PHLPPL'' gene In biology, the word gene (from , ; "...Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a b .... See also * PHLPP References Further reading * * * * * * * * * * * {{gene-16-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Kinase C
In cell biology, Protein kinase C, commonly abbreviated to PKC (EC 2.7.11.13), is a family of protein kinase enzymes that are involved in controlling the function of other proteins through the phosphorylation of hydroxyl groups of serine and threonine amino acid residues on these proteins, or a member of this family. PKC enzymes in turn are activated by signals such as increases in the concentration of diacylglycerol (DAG) or calcium ions (Ca2+). Hence PKC enzymes play important roles in several signal transduction cascades. In biochemistry, the PKC family consists of fifteen isozymes in humans. They are divided into three subfamilies, based on their second messenger requirements: conventional (or classical), novel, and atypical. Conventional (c)PKCs contain the isoforms α, βI, βII, and γ. These require Ca2+, DAG, and a phospholipid such as phosphatidylserine for activation. Novel (n)PKCs include the δ, ε, η, and θ isoforms, and require DAG, but do not require Ca2+ for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pleckstrin Homology Domain
Pleckstrin homology domain (PH domain) or (PHIP) is a protein domain of approximately 120 amino acids that occurs in a wide range of proteins involved in intracellular signaling or as constituents of the cytoskeleton. This domain can bind phosphatidylinositol lipids within biological membranes (such as phosphatidylinositol (3,4,5)-trisphosphate and phosphatidylinositol (4,5)-bisphosphate), and proteins such as the βγ-subunits of heterotrimeric G proteins, and protein kinase C. Through these interactions, PH domains play a role in recruiting proteins to different membranes, thus targeting them to appropriate cellular compartments or enabling them to interact with other components of the signal transduction pathways. Lipid binding specificity Individual PH domains possess specificities for phosphoinositides phosphorylated at different sites within the inositol ring, e.g., some bind phosphatidylinositol (4,5)-bisphosphate but not phosphatidylinositol (3,4,5)-trisphosphate or p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Loss Of Heterozygosity
Loss of heterozygosity (LOH) is a type of genetic abnormality in diploid organisms in which one copy of an entire gene and its surrounding chromosomal region are lost. Since diploid cells have two copies of their genes, one from each parent, a single copy of the lost gene still remains. But any heterozygosity, slight differences between the versions of the gene inherited from each parent, is no longer present. In cancer The loss of heterozygosity is a common occurrence in cancer development. Originally, a heterozygous state is required and indicates the absence of a functional tumor suppressor gene copy in the region of interest. However, many people remain healthy with such a loss, because there still is one functional gene left on the other chromosome of the chromosome pair. The remaining copy of the tumor suppressor gene can be inactivated by a point mutation or via other mechanisms, resulting in a loss of heterozygosity event, and leaving no tumor suppressor gene to protect the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G Protein-coupled Receptor
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily-related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. Coupling with G proteins, they are called seven-transmembrane receptors because they pass through the cell membrane seven times. Text was copied from this source, which is available under Attribution 2.5 Generic (CC BY 2.5) license. Ligands can bind either to extracellular N-terminus and loops (e.g. glutamate receptors) or to the binding site within transmembrane helices (Rhodopsin-like family). They are all activated by agonists although a spontaneous auto-activation of an empty receptor can also be observed. G protein-coupled receptors are found only in eukaryotes, including yeast, choanoflagellates, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diacylglycerol
A diglyceride, or diacylglycerol (DAG), is a glyceride consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages. Two possible forms exist, 1,2-diacylglycerols and 1,3-diacylglycerols. DAGs can act as surfactants and are commonly used as emulsifiers in processed foods. DAG-enriched oil (particularly 1,3-DAG) has been investigated extensively as a fat substitute due to its ability to suppress the accumulation of body fat; with total annual sales of approximately USD 200 million in Japan since its introduction in the late 1990s till 2009. Production Diglycerides are a minor component of many seed oils and are normally present at ~1–6%; or in the case of cottonseed oil as much as 10%. Industrial production is primarily achieved by a glycerolysis reaction between triglycerides and glycerol. The raw materials for this may be either vegetable oils or animal fats. Food additive Diglycerides, generally in a mix with monoglycerides (E471), a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphomimetics
Phosphomimetics are amino acid substitutions that mimic a phosphorylated protein, thereby activating (or deactivating) the protein. Within cells, proteins are commonly modified at serine, tyrosine and threonine amino acids by adding a phosphate group. Phosphorylation is a common mode of activating or deactivating a protein as a form of regulation. However some non-phosphorylated amino acids appear chemically similar to phosphorylated amino acids. Therefore, by replacing an amino acid, the protein may maintain a higher level of activity. For example, aspartic acid is chemically similar to phospho-serine. Therefore, when an aspartic acid replaces a serine, it is a phosphomimetic of phospho-serine and can make the protein always in its phosphorylated form. Phosphonate-based compounds have been used as phosphotyrosine analogues, as they are less enzyme labile and are physiologically more stable. Applications This chemical similarity can be exploited in cancer, where a protein may ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activation Loop
In molecular biology, an intrinsically disordered protein (IDP) is a protein that lacks a fixed or ordered three-dimensional structure, typically in the absence of its macromolecular interaction partners, such as other proteins or RNA. IDPs range from fully unstructured to partially structured and include random coil, molten globule-like aggregates, or flexible linkers in large multi-domain proteins. They are sometimes considered as a separate class of proteins along with globular, fibrous and membrane proteins. IDPs are a very large and functionally important class of proteins and their discovery has disproved the idea that three-dimensional structures of proteins must be fixed to accomplish their biological functions. For example, IDPs have been identified to participate in weak multivalent interactions that are highly cooperative and dynamic, lending them importance in DNA regulation and in cell signaling. Many IDPs can also adopt a fixed three-dimensional structure after bi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Synthesis
Protein biosynthesis (or protein synthesis) is a core biological process, occurring inside Cell (biology), cells, homeostasis, balancing the loss of cellular proteins (via Proteolysis, degradation or Protein targeting, export) through the production of new proteins. Proteins perform a number of critical functions as enzymes, structural proteins or hormones. Protein synthesis is a very similar process for both prokaryotes and eukaryotes but there are some distinct differences. Protein synthesis can be divided broadly into two phases - Transcription (biology), transcription and Translation (biology), translation. During transcription, a section of DNA encoding a protein, known as a gene, is converted into a template molecule called messenger RNA (mRNA). This conversion is carried out by enzymes, known as RNA polymerases, in the cell nucleus, nucleus of the cell. In eukaryotes, this mRNA is initially produced in a premature form (Primary transcript, pre-mRNA) which undergoes post-tra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PIP3
Phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)''P''3), abbreviated PIP3, is the product of the class I phosphoinositide 3-kinases (PI 3-kinases) phosphorylation of phosphatidylinositol (4,5)-bisphosphate (PIP2). It is a phospholipid that resides on the plasma membrane. Discovery In 1988, Lewis C. Cantley published a paper describing the discovery of a novel type of phosphoinositide kinase with the unprecedented ability to phosphorylate the 3' position of the inositol ring resulting in the formation of phosphatidylinositol-3-phosphate (PI3P). Working independently, Alexis Traynor-Kaplan and coworkers published a paper demonstrating that a novel lipid, phosphatidylinositol 3,4,5 trisphosphate (PIP3) occurs naturally in human neutrophils with levels that increased rapidly following physiologic stimulation with chemotactic peptide. Subsequent studies demonstrated that ''in vivo'' the enzyme originally identified by Cantley's group prefers PtdIns(4,5)P2 as a substrate, pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Insulin Receptor
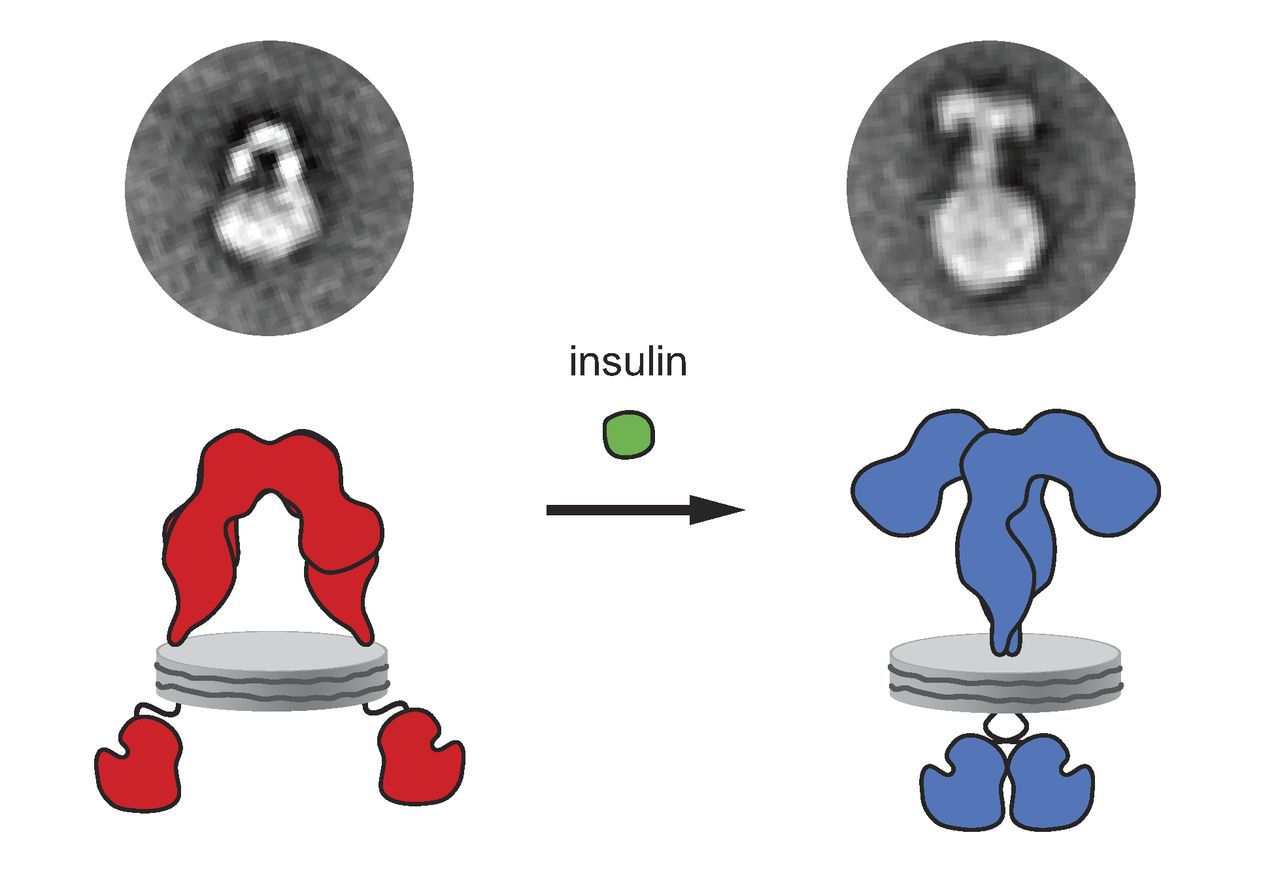
The insulin receptor (IR) is a transmembrane receptor that is activated by insulin, IGF-I, IGF-II and belongs to the large class of receptor tyrosine kinase. Metabolically, the insulin receptor plays a key role in the regulation of glucose homeostasis, a functional process that under degenerate conditions may result in a range of clinical manifestations including diabetes and cancer. Insulin signalling controls access to blood glucose in body cells. When insulin falls, especially in those with high insulin sensitivity, body cells begin only to have access to lipids that do not require transport across the membrane. So, in this way, insulin is the key regulator of fat metabolism as well. Biochemically, the insulin receptor is encoded by a single gene , from which alternate splicing during transcription results in either IR-A or IR-B isoforms. Downstream post-translational events of either isoform result in the formation of a proteolytically cleaved α and β subunit, which upon com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |