|
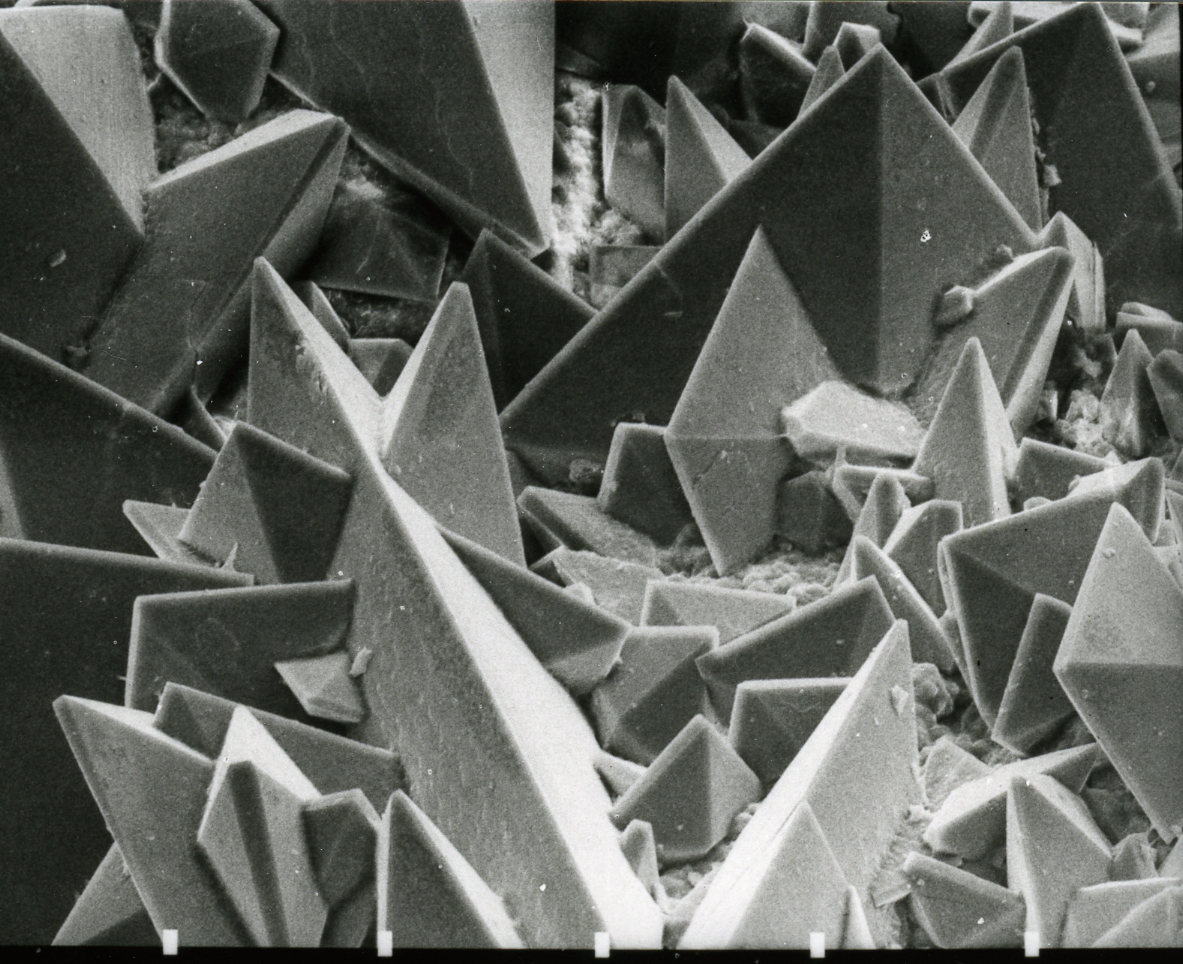
Potassium Ferrioxalate
Potassium ferrioxalate, also called potassium trisoxalatoferrate or potassium tris(oxalato)ferrate(III) is a chemical compound with the formula []. It often occurs as the trihydrate . Both are crystalline compounds, lime green in colour.A. Saritha, B. Raju, M. Ramachary, P. Raghavaiah, and K. A. Hussain (2012) "Synthesis, crystal structure and characterization of chiral, three-dimensional anhydrous potassium tris(oxalato)ferrate(III)", ''Physica B: Condensed Matter'', volume 407, issue 21, pages 4208-4213. The compound is a salt consisting of ferrioxalate anions, , and potassium cations . The anion is a transition metal complex consisting of an iron atom in the +3 oxidation state and three bidentate oxalate ions anions acting as ligands. Potassium acts as a counterion, balancing the −3 charge of the complex. In solution, the salt dissociates to give the ferrioxalate anion, []3−, which appears fluorescent green in color. The ferrioxalate anion is quite stable in the da ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octahedral
In geometry, an octahedron (plural: octahedra, octahedrons) is a polyhedron with eight faces. The term is most commonly used to refer to the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet at each vertex. A regular octahedron is the dual polyhedron of a cube. It is a rectified tetrahedron. It is a square bipyramid in any of three orthogonal orientations. It is also a triangular antiprism in any of four orientations. An octahedron is the three-dimensional case of the more general concept of a cross polytope. A regular octahedron is a 3-ball in the Manhattan () metric. Regular octahedron Dimensions If the edge length of a regular octahedron is ''a'', the radius of a circumscribed sphere (one that touches the octahedron at all vertices) is :r_u = \frac a \approx 0.707 \cdot a and the radius of an inscribed sphere (tangent to each of the octahedron's faces) is :r_i = \frac a \approx 0.408\cdot a while the midradius, which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actinometry
Actinometers are instruments used to measure the heating power of radiation. They are used in meteorology to measure solar radiation as pyranometers, pyrheliometers and net radiometers. An actinometer is a chemical system or physical device which determines the number of photons in a beam integrally or per unit time. This name is commonly applied to devices used in the ultraviolet and visible wavelength ranges. For example, solutions of iron(III) oxalate can be used as a chemical actinometer, while bolometers, thermopiles, and photodiodes are physical devices giving a reading that can be correlated to the number of photons detected. History The actinometer was invented by John Herschel in 1825; he introduced the term ''actinometer'', the first of many uses of the prefix ''actin'' for scientific instruments, effects, and processes. The actinograph is a related device for estimating the actinic power of lighting for photography. Chemical actinometry Chemical actinometry invol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth's atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm. Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.IPCC (2022Summary for policy makersiClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in groundwater, lakes, i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gamma Rays
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically shorter than those of X-rays. With frequencies above 30 exahertz (), it imparts the highest photon energy. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900 while studying radiation emitted by radium. In 1903, Ernest Rutherford named this radiation ''gamma rays'' based on their relatively strong penetration of matter; in 1900 he had already named two less penetrating types of decay radiation (discovered by Henri Becquerel) alpha rays and beta rays in ascending order of penetrating power. Gamma rays from radioactive decay are in the energy range from a few kiloelectronvolts ( keV) to approximately 8 megaelectronvolts ( MeV), corresponding to the typical energy levels in nuclei with reasonably long lifeti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-rays
X-rays (or rarely, ''X-radiation'') are a form of high-energy electromagnetic radiation. In many languages, it is referred to as Röntgen radiation, after the German scientist Wilhelm Conrad Röntgen, who discovered it in 1895 and named it ''X-radiation'' to signify an unknown type of radiation.Novelline, Robert (1997). ''Squire's Fundamentals of Radiology''. Harvard University Press. 5th edition. . X-ray wavelengths are shorter than those of ultraviolet rays and longer than those of gamma rays. There is no universally accepted, strict definition of the bounds of the X-ray band. Roughly, X-rays have a wavelength ranging from 10 nanometers to 10 picometers, corresponding to frequencies in the range of 30 petahertz to 30 exahertz ( to ) and photon energies in the range of 100 eV to 100 keV, respectively. X-rays can penetrate many solid substances such as construction materials and living tissue, so X-ray radiography is widely used in medic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helical Chirality
Helical may refer to: * Helix A helix () is a shape like a corkscrew or spiral staircase. It is a type of smooth space curve with tangent lines at a constant angle to a fixed axis. Helices are important in biology, as the DNA molecule is formed as two intertwined hel ..., the mathematical concept for the shape * Helical engine, a proposed spacecraft propulsion drive * Helical spring, a coilspring * Helical plc, a British property company, once a maker of steel bar stock * Helicoil, a mechanical thread repairing insert * H-el-ical//, stage name for Hikaru, Japanese singer {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomorphous
In mathematics, an isomorphism is a structure-preserving mapping between two structures of the same type that can be reversed by an inverse mapping. Two mathematical structures are isomorphic if an isomorphism exists between them. The word isomorphism is derived from the Ancient Greek: ἴσος ''isos'' "equal", and μορφή ''morphe'' "form" or "shape". The interest in isomorphisms lies in the fact that two isomorphic objects have the same properties (excluding further information such as additional structure or names of objects). Thus isomorphic structures cannot be distinguished from the point of view of structure only, and may be identified. In mathematical jargon, one says that two objects are . An automorphism is an isomorphism from a structure to itself. An isomorphism between two structures is a canonical isomorphism (a canonical map that is an isomorphism) if there is only one isomorphism between the two structures (as it is the case for solutions of a unive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
High Spin
Spin states when describing transition metal coordination complexes refers to the potential spin configurations of the central metal's d electrons. For several oxidation states, metals can adopt high-spin and low-spin configurations. The ambiguity only applies to first row metals, because second- and third-row metals are invariably low-spin. These configurations can be understood through the two major models used to describe coordination complexes; crystal field theory and ligand field theory (a more advanced version based on molecular orbital theory). High-spin vs. low-spin Octahedral complexes The Δ splitting of the ''d'' orbitals plays an important role in the electron spin state of a coordination complex. Three factors affect Δ: the period (row in periodic table) of the metal ion, the charge of the metal ion, and the field strength of the complex's ligands as described by the spectrochemical series. Only octahedral complexes of first row transition metals adopt high ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Syntheses
''Inorganic Syntheses'' is a book series which aims to publish "detailed and foolproof" procedures for the synthesis of inorganic compounds. Although this series of books are edited, they usually are referenced like a journal, without mentioning the names of the checkers (referees) or the editor. A similar format is usually followed for the series '' Organic Syntheses''. Volumes See also * Organic SynthesesReferences {{chem-book-stub Boo ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Oxalate
Oxalate (IUPAC: ethanedioate) is an anion with the formula C2O42−. This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salt (chemistry), salts, for example sodium oxalate (Na2C2O4), and several esters such as dimethyl oxalate (C2O4(CH3)2). It is a conjugate base of oxalic acid. At neutral pH in aqueous solution, oxalic acid converts completely to oxalate. Relationship to oxalic acid The dissociation of protons from oxalic acid proceeds in a stepwise manner; as for other acid#Polyprotic acids, polyprotic acids, loss of a single proton results in the monovalent hydrogenoxalate anion . A salt (chemistry), salt with this anion is sometimes called an acid oxalate, monobasic oxalate, or hydrogen oxalate. The equilibrium constant (acid dissociation constant, ''K''a) for loss of the first proton is (p''K''a = 1.27). The loss of the second proton, which yields the oxalate ion, has an equilibrium constant of (p''K''a = 4.28) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |