|
Plasma-enhanced Chemical Vapor Deposition
Plasma-enhanced chemical vapor deposition (PECVD) is a chemical vapor deposition process used to deposit thin films from a gas state (vapor) to a solid state on a substrate. Chemical reactions are involved in the process, which occur after creation of a plasma of the reacting gases. The plasma is generally created by radio frequency (RF) (alternating current (AC)) frequency or direct current (DC) discharge between two electrodes, the space between which is filled with the reacting gases. Discharges for processes A plasma is any gas in which a significant percentage of the atoms or molecules are ionized. Fractional ionization in plasmas used for deposition and related materials processing varies from about 10−4 in typical capacitive discharges to as high as 5–10% in high-density inductive plasmas. Processing plasmas are typically operated at pressures of a few millitorrs to a few torr, although arc discharges and inductive plasmas can be ignited at atmospheric pressure. Pla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
STS Multiplex PECVD Machine At LAAS 0495
STS, or sts, may refer to: Medicine * Secondary traumatic stress, a condition which leads to a diminished ability to empathize * Sequence-tagged site, a gene-reference in genomics * Soft-tissue sarcoma * Staurosporine, an antibiotic * STS (gene), which codes for steroid sulfatase * Superior temporal sulcus Places * Semipalatinsk Test Site for Soviet nuclear weapons * Staffordshire, county in England, Chapman code Transport * Cadillac STS, a luxury car * NASA Space Transportation System, the system in which the NASA shuttle is part of and only surviving component of; starting as a 1969 NASA proposal system for reusable space vehicles ** NASA Space Shuttle program, the shuttle program itself, whose mission were referred to with STS-numbering * Sail training ship, a ship prefix * Satellite Transit System, now called the SEA Underground, airport transit in Seattle-Tacoma International Airport * Ship-to-ship transfer, between seagoing ships * ''Société de transport de Sherbrook ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids ( DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere. Many indus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is both caust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
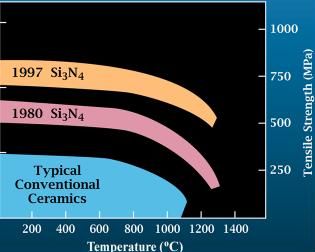
Silicon Nitride
Silicon nitride is a chemical compound of the elements silicon and nitrogen. is the most thermodynamically stable and commercially important of the silicon nitrides, and the term "silicon nitride" commonly refers to this specific composition. It is a white, high-melting-point solid that is relatively chemically inert, being attacked by dilute HF and hot . It is very hard (8.5 on the mohs scale). It has a high thermal stability with strong optical nonlinearities for all-optical applications. Production Silicon nitride is prepared by heating powdered silicon between 1300 °C and 1400 °C in a nitrogen atmosphere: :3 Si + 2 → The silicon sample weight increases progressively due to the chemical combination of silicon and nitrogen. Without an iron catalyst, the reaction is complete after several hours (~7), when no further weight increase due to nitrogen absorption (per gram of silicon) is detected. In addition to , several other silicon nitride phases (with chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrous Oxide
Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas, nitrous, or nos, is a chemical compound, an oxide of nitrogen with the formula . At room temperature, it is a colourless non-flammable gas, and has a slightly sweet scent and taste. At elevated temperatures, nitrous oxide is a powerful oxidiser similar to molecular oxygen. Nitrous oxide has significant medical uses, especially in surgery and dentistry, for its anaesthetic and pain-reducing effects. Its colloquial name, "laughing gas", coined by Humphry Davy, is due to the euphoric effects upon inhaling it, a property that has led to its recreational use as a dissociative anaesthetic. It is on the World Health Organization's List of Essential Medicines. It is also used as an oxidiser in rocket propellants, and in motor racing to increase the power output of engines. Nitrous oxide's atmospheric concentration reached 333 parts per billion (ppb) in 2020, increasing at a rate of abo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats, as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silane
Silane is an inorganic compound with chemical formula, . It is a colourless, pyrophoric, toxic gas with a sharp, repulsive smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon. Silane with alkyl groups are effective water repellents for mineral surfaces such as concrete and masonry. Silanes with both organic and inorganic attachments are used as coupling agents. Production Commercial-scale routes Silane can be produced by several routes. Typically, it arises from the reaction of hydrogen chloride with magnesium silicide: : Mg2Si + 4 HCl -> 2 MgCl2 + SiH4 It is also prepared from metallurgical-grade silicon in a two-step process. First, silicon is treated with hydrogen chloride at about 300 °C to produce trichlorosilane, HSiCl3, along with hydrogen gas, according to the chemical equation : Si + 3 HCl -> HSiCl3 + H2 The trichlorosilane is then converted to a mixture of silane and silicon tetrachloride: : 4 HS ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dichlorosilane
Dichlorosilane, or DCS as it is commonly known, is a chemical compound with the formula H2SiCl2. In its major use, it is mixed with ammonia (NH3) in LPCVD chambers to grow silicon nitride in semiconductor processing. A higher concentration of DCS·NH3 (i.e. 16:1), usually results in lower stress nitride films. History Dichlorosilane was originally prepared in 1919 by the gas-phase reaction of monosilane, SiH4, with hydrogen chloride, HCl, and then reported by Stock and Somieski. It was found that in the gas phase, dichlorosilane will react with water vapor to give a gaseous monomeric prosiloxane, H2SiO. Prosiloxane polymerizes rapidly in the liquid phase and slowly in the gas phase, which results in liquid and solid polysiloxanes 2SiOsub>n. The liquid portion of the product, which is collected via vacuum distillation, becomes viscous and gelled at room temperature. Hydrolysis was done on a solution of H2SiCl2 in benzene by brief contact with water, and the molecular weight was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon Dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and most abundant families of materials, existing as a compound of several minerals and as a synthetic product. Notable examples include fused quartz, fumed silica, silica gel, opal and aerogels. It is used in structural materials, microelectronics (as an electrical insulator), and as components in the food and pharmaceutical industries. Structure In the majority of silicates, the silicon atom shows tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. In contrast, CO2 is a linear molecule. The starkly different structures of the dioxid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
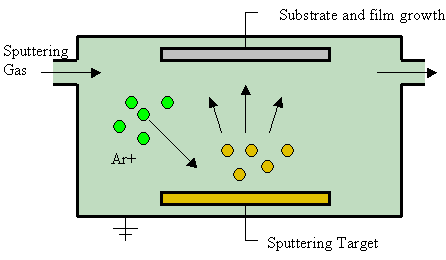
Sputter Deposition
Sputter deposition is a physical vapor deposition (PVD) method of thin film deposition by the phenomenon of sputtering. This involves ejecting material from a "target" that is a source onto a "substrate" such as a silicon wafer. Resputtering is re-emission of the deposited material during the deposition process by ion or atom bombardment. Sputtered atoms ejected from the target have a wide energy distribution, typically up to tens of eV (100,000 K). The sputtered ions (typically only a small fraction of the ejected particles are ionized — on the order of 1 percent) can ballistically fly from the target in straight lines and impact energetically on the substrates or vacuum chamber (causing resputtering). Alternatively, at higher gas pressures, the ions collide with the gas atoms that act as a moderator and move diffusively, reaching the substrates or vacuum chamber wall and condensing after undergoing a random walk. The entire range from high-energy ballistic impact to low-energy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


-3D-balls.png)