|
Plant Communication
Plants are exposed to many stress factors such as disease, temperature changes, herbivory, injury and more. Therefore, in order to respond or be ready for any kind of physiological state, they need to develop some sort of system for their survival in the moment and/or for the future. Plant communication encompasses communication using volatile organic compounds, electrical signaling, and common mycorrhizal networks between plants and a host of other organisms such as soil microbes, other plants (of the same or other species), animals, insects, and fungi. Plants communicate through a host of volatile organic compounds (VOCs) that can be separated into four broad categories, each the product of distinct chemical pathways: fatty acid derivatives, phenylpropanoids/benzenoids, amino acid derivatives, and terpenoids. Due to the physical/chemical constraints most VOCs are of low molecular mass (< 300 Da), are hydrophobic, and have high vapor pressures. The responses of organisms to plant e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Soil Microbe
Soil microbiology is the study of microorganisms in soil, their functions, and how they affect soil properties. It is believed that between two and four billion years ago, the first ancient bacteria and microorganisms came about on Earth's oceans. These bacteria could Nitrogen fixation, fix nitrogen, in time fission (biology)#Binary fission, multiplied, and as a result released oxygen into the atmosphere. This led to more advanced microorganisms, which are important because they affect soil structure and fertility. Soil microorganisms can be classified as bacteria, Actinomycetota, actinomycetes, Fungus, fungi, algae and protozoa. Each of these groups has characteristics that define them and their functions in soil.Rao, Subba. Soil Microbiology. Fourth ed. Enfield: Science Publishers, 1999. Print. Up to 10 billion bacterial cells inhabit each gram of soil in and around plant roots, a region known as the rhizosphere. In 2011, a team detected more than 33,000 bacterial and archaeal spe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Green Leaf Volatile
Green leaf volatiles (GLV) are organic compounds released by plants. Some of these chemicals function as signaling compounds between either plants of the same species, of other species, or even different lifeforms like insects. Green leaf volatiles are involved in patterns of attack and protection between species. They have been found to increase the attractive effect of pheromones of cohabiting insect species that protect plants from attacking insect species. For example, corn plants that are being fed on by caterpillars will release GLVs that attract wasps, who then attack the caterpillars. GLVs also have antimicrobial properties that can prevent infection at the site of injury. GLVs include C6-aldehydes Z)-3-hexenal, n-hexanaland their derivatives such as (Z)-3-hexenol, (Z)-3-hexen-1-yl acetate, and the corresponding E-isomers. Functions When a plant is attacked, it emits GLVs into the environment through the air. How a plant responds depends on the type of damage involv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
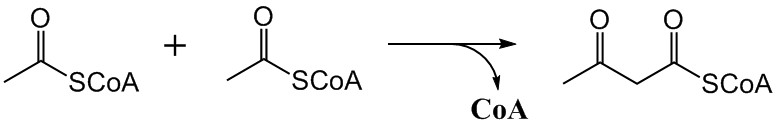
Mevalonate Pathway
The mevalonate pathway, also known as the isoprenoid pathway or HMG-CoA reductase pathway is an essential metabolic pathway present in eukaryotes, archaea, and some bacteria. The pathway produces two five-carbon building blocks called isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which are used to make isoprenoids, a diverse class of over 30,000 biomolecules such as cholesterol, vitamin K, coenzyme Q10, and all steroid hormones. The mevalonate pathway begins with acetyl-CoA and ends with the production of IPP and DMAPP. It is best known as the target of statins, a class of cholesterol lowering drugs. Statins inhibit HMG-CoA reductase within the mevalonate pathway. Upper mevalonate pathway The mevalonate pathway of eukaryotes, archaea, and eubacteria all begin the same way. The sole carbon feed stock of the pathway is acetyl-CoA. The first step condenses two acetyl-CoA molecules to yield acetoacetyl-CoA. This is followed by a second condensation to form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-mevalonate Pathway
The non-mevalonate pathway—also appearing as the mevalonate-independent pathway and the 2-''C''-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate (MEP/DOXP) pathway—is an alternative metabolic pathway for the biosynthesis of the isoprenoid precursors isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). The currently preferred name for this pathway is the MEP pathway, since MEP is the first committed metabolite on the route to IPP. Isoprenoid precursor biosynthesis The mevalonate pathway (MVA pathway or HMG-CoA reductase pathway) and the MEP pathway are metabolic pathways for the biosynthesis of isoprenoid precursors: IPP and DMAPP. Whereas plants use both MVA and MEP pathway, most organisms only use one of the pathways for the biosynthesis of isoprenoid precursors. In plant cells IPP/DMAPP biosynthesis via the MEP pathway takes place in plastid organelles, while the biosynthesis via the MVA pathway takes place in the cytoplasm. Most gram-nega ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cellular And Molecular Life Sciences
Cellular may refer to: * Cellular automaton, a model in discrete mathematics *Cell biology, the evaluation of cells work and more * ''Cellular'' (film), a 2004 movie *Cellular frequencies, assigned to networks operating in cellular RF bands * Cellular manufacturing *Cellular network, cellular radio networks * U.S. Cellular Field, also known as "The Cell", a baseball stadium in Chicago * U.S. Cellular Arena, an arena in Milwaukee, Wisconsin Terms such as cellular organization, cellular structure, cellular system, and so on may refer to: *Cell biology, the evaluation of how cells work and more * Cellular communication networks, systems for allowing communication through mobile phones and other mobile devices *Cellular organizational structures A non-biological entity with a cellular organizational structure (also known as a cellular organization, cellular system, nodal organization, nodal structure, et cetera) is set up in such a way that it mimics how Natural science, natural ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoprenoid
The terpenoids, also known as isoprenoids, are a class of naturally occurring organic chemicals derived from the 5-carbon compound isoprene and its derivatives called terpenes, diterpenes, etc. While sometimes used interchangeably with "terpenes", terpenoids contain additional functional groups, usually containing oxygen. When combined with the hydrocarbon terpenes, terpenoids comprise about 80,000 compounds. They are the largest class of plant secondary metabolites, representing about 60% of known natural products. Many terpenoids have substantial pharmacological bioactivity and are therefore of interest to medicinal chemists. Plant terpenoids are used for their aromatic qualities and play a role in traditional herbal remedies. Terpenoids contribute to the scent of eucalyptus, the flavors of cinnamon, cloves, and ginger, the yellow color in sunflowers, and the red color in tomatoes. Well-known terpenoids include citral, menthol, camphor, salvinorin A in the plant '' S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biogenetic Isoprene Rule
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Terpenes are major biosynthetic building blocks. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. In plants, terpenes and terpenoids are important mediators of ecological interactions, while some insects use some terpenes as a form of defense. Other functions of terpenoids include cell growth modulation and plant elongation, light harvesting and photoprotection, and membrane permeability and fluidity control. Terpenes are classified by the number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), as examples. The terpene alpha-pinene is a major component of the common solvent, turpentine. The one terpene that has major applications is natural rubber (i.e., polyisoprene). The possibility that other terpenes could be used as precursors to produce synthetic polymers has been ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The Reactivity (chemistry), reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive Chemical property, chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their Chemical polarity, nonp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terpene
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Terpenes are major biosynthetic building blocks. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly Pinophyta, conifers. In plants, terpenes and terpenoids are important mediators of ecological biological interaction, interactions, while some insects use some terpenes as a form of defense. Other functions of terpenoids include cell growth modulation and plant elongation, light harvesting and photoprotection, and membrane permeability and fluidity control. Terpenes are classified by the number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), as examples. The terpene alpha-pinene is a major component of the common solvent, turpentine. The one terpene that has major applications is natural rubber (i.e., polyisoprene). The possibility that other terpenes could be used as precursors to pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Verbenone
Verbenone is a natural organic compound classified as a terpene that is found naturally in a variety of plants. The chemical has a pleasant characteristic odor. Besides being a natural constituent of plants, it and its analogs are insect pheromones. In particular, verbenone when formulated in a long-lasting matrix has an important role in the control of bark beetles such as the mountain pine beetle and the Southern pine bark beetle. Chemistry Verbenone is a monoterpene, to be specific a bicyclic ketone terpene. It is the primary constituent of the oil of Spanish verbena, hence its name; it is also found in the oil of rosemary. It is nearly insoluble in water, but miscible with most organic solvents. Verbenone can be readily prepared synthetically by the oxidation of the more common terpene α-pinene: : Verbenone can then be converted into chrysanthenone through a photochemical rearrangement reaction: : Use for insect control The southern pine beetle ('' Dendroctonus fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scots Pine
''Pinus sylvestris'', the Scots pine (UK), Scotch pine (US), Baltic pine, or European red pine is a species of tree in the pine family Pinaceae that is native to Eurasia. It can readily be identified by its combination of fairly short, blue-green leaves and orange-red bark. Description ''Pinus sylvestris'' is an evergreen coniferous tree growing up to in height and in trunk diameter when mature, exceptionally over tall and in trunk diameter on very productive sites. The tallest on record is a tree over 210 years old growing in Estonia which stands at . The lifespan is normally 150–300 years, with the oldest recorded specimens in Lapland, Northern Finland over 760 years. The bark is thick, flaky and orange-red when young to scaly and gray-brown in maturity, sometimes retaining the former on the upper portion. The habit of the mature tree is distinctive due to its long, bare and straight trunk topped by a rounded or flat-topped mass of foliage. The shoots are light ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |