|
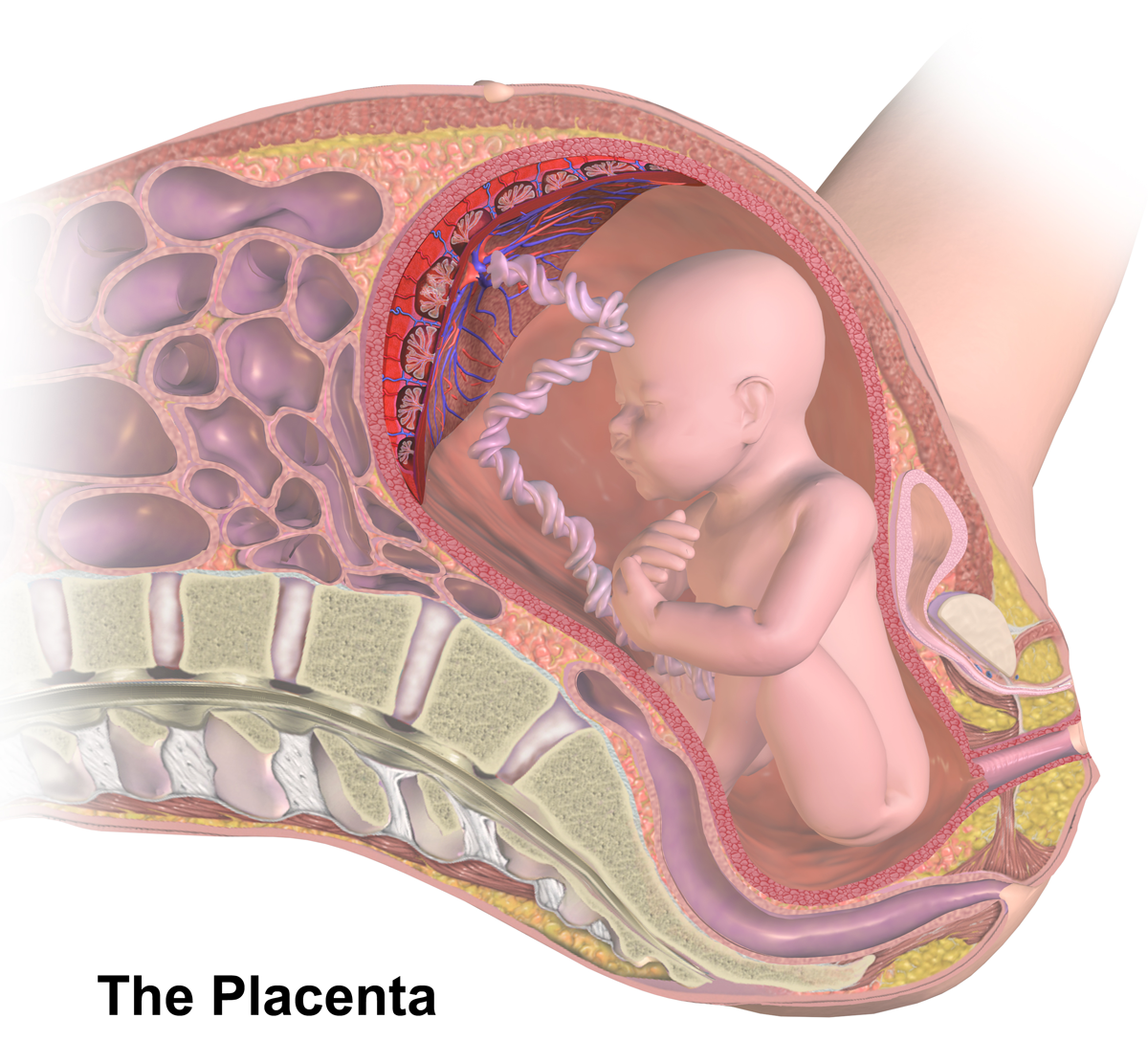
Placental Insufficiency
Placental insufficiency or utero-placental insufficiency is the failure of the placenta to deliver sufficient nutrients to the fetus during pregnancy, and is often a result of insufficient blood flow to the placenta. The term is also sometimes used to designate late decelerations of fetal heart rate as measured by cardiotocography or an NST, even if there is no other evidence of reduced blood flow to the placenta, normal uterine blood flow rate being 600mL/min. Causes The following characteristics of placentas have been said to be associated with placental insufficiency, however all of them occur in normal healthy placentas and full term healthy births, so none of them can be used to accurately diagnose placental insufficiency: * Abnormally thin placenta (less than 1 cm) * Circumvallate placenta (1% of normal placentas) * Amnion cell metaplasia, ( amnion nodosum) (present in 65% of normal placentas) * Increased syncytial knots * Calcifications * Infarcts due to focal or diff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Placenta
The placenta is a temporary embryonic and later fetal organ that begins developing from the blastocyst shortly after implantation. It plays critical roles in facilitating nutrient, gas and waste exchange between the physically separate maternal and fetal circulations, and is an important endocrine organ, producing hormones that regulate both maternal and fetal physiology during pregnancy. The placenta connects to the fetus via the umbilical cord, and on the opposite aspect to the maternal uterus in a species-dependent manner. In humans, a thin layer of maternal decidual (endometrial) tissue comes away with the placenta when it is expelled from the uterus following birth (sometimes incorrectly referred to as the 'maternal part' of the placenta). Placentas are a defining characteristic of placental mammals, but are also found in marsupials and some non-mammals with varying levels of development. Mammalian placentas probably first evolved about 150 million to 200 million years ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight, where it is used to make cellulose in cell walls, the most abundant carbohydrate in the world. In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is stored as a polymer, in plants mainly as starch and amylopectin, and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form of glucose is -glucose, while -glucose is produced synthetically in comparatively small amounts and is less biologically active. Glucose is a monosaccharide containing six carbon atoms and an aldehyde group, and is therefore an aldohexose. The glucose molecule can exist in an open-chain (acyclic) as well as ring (cyclic) form. Gluco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyunsaturated Fatty Acids
Polyunsaturated fatty acids (PUFAs) are fatty acids that contain more than one double bond in their backbone. This class includes many important compounds, such as essential fatty acids and those that give drying oils their characteristic property. Polyunsaturated fatty acids can be classified in various groups by their chemical structure: * methylene-interrupted polyenes * conjugated fatty acids * other PUFAs Based on the length of their carbon backbone, they are sometimes classified in two groups: * short chain polyunsaturated fatty acids (SC-PUFA), with 18 carbon atoms * long-chain polyunsaturated fatty acids (LC-PUFA) with 20 or more carbon atoms Dietary sources Types Methylene-interrupted polyenes These fatty acids have 2 or more ''cis'' double bonds that are separated from each other by a single methylene bridge (--). This form is also sometimes called a ''divinylmethane pattern''. The essential fatty acids are all omega-3 and -6 methylene-interrupted fatty ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fatty Acid
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are a major component of the lipids (up to 70% by weight) in some species such as microalgae but in some other organisms are not found in their standalone form, but instead exist as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important dietary sources of fuel for animals and important structural components for cells. History The concept of fatty acid (''acide gras'') was introduced in 1813 by Michel Eugène Chevreul, though he initially used some variant terms: ''graisse acide'' and ''acide huileux'' ("acid fat" and "oily acid"). Types of fatty acids Fatty acids are classified in many ways: by length, by saturation vs unsaturati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ponderal Index
The Corpulence Index (CI) (also Ponderal Index (PI) or Rohrer's Index) is a measure of corpulence, or of leanness in other variants, of a person''Foods and Nutrition Encyclopedia'', Audrey H. Ensminger, Marion Eugene Ensminger. p. 1645 calculated as a relationship between mass and height.EXSS 323: LAB 1 - BIOMECHANICS TOOLS: Computers, Algebra and Trig Oregon State University It was first proposed in 1921 as the "Corpulence measure" by Swiss physician Fritz Rohrer and hence is also known as Rohrer's Index. It is similar to the body mass index, but the mass is normalized with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is both caust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid (carbamic acid is unstable), with the chemical formula NH2‐ CH2‐ COOH. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (GGU, GGC, GGA, GGG). Glycine is integral to the formation of alpha-helices in secondary protein structure due to its compact form. For the same reason, it is the most abundant amino acid in collagen triple-helices. Glycine is also an inhibitory neurotransmitter – interference with its release within the spinal cord (such as during a ''Clostridium tetani'' infection) can cause spastic paralysis due to uninhibited muscle contraction. It is the only achiral proteinogenic amino acid. It can fit into hydrophilic or hydrophobic environments, due to its minimal side chain of only one hydrogen atom. History and etymology Glycine was discovered in 1820 by the French chemist He ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
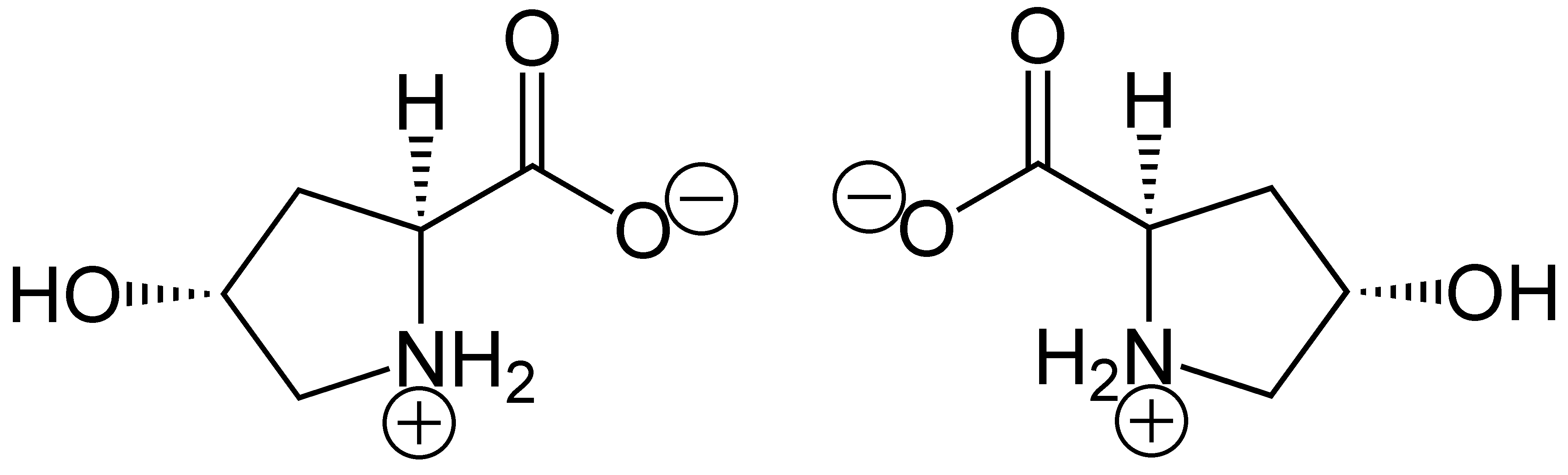
Hydroxyproline
(2''S'',4''R'')-4-Hydroxyproline, or L-hydroxyproline ( C5 H9 O3 N), is an amino acid, abbreviated as Hyp or O, ''e.g.'', in Protein Data Bank. Structure and discovery In 1902, Hermann Emil Fischer isolated hydroxyproline from hydrolyzed gelatin. In 1905, Hermann Leuchs synthesized a racemic mixture of 4-hydroxyproline. Hydroxyproline differs from proline by the presence of a hydroxyl (OH) group attached to the gamma carbon atom. Production and function Hydroxyproline is produced by hydroxylation of the amino acid proline by the enzyme prolyl hydroxylase following protein synthesis (as a post-translational modification). The enzyme catalyzed reaction takes place in the lumen of the endoplasmic reticulum. Although it is not directly incorporated into proteins, hydroxyproline comprises roughly 4% of all amino acids found in animal tissue, an amount greater than seven other amino acids that are translationally incorporated. Animals Collagen Hydroxyproline is a major compon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain lysyl ((CH2)4NH2), classifying it as a basic, charged (at physiological pH), aliphatic amino acid. It is encoded by the codons AAA and AAG. Like almost all other amino acids, the α-carbon is chiral and lysine may refer to either enantiomer or a racemic mixture of both. For the purpose of this article, lysine will refer to the biologically active enantiomer L-lysine, where the α-carbon is in the ''S'' configuration. The human body cannot synthesize lysine. It is essential in humans and must therefore be obtained from the diet. In organisms that synthesise lysine, two main biosynthetic pathways exist, the diaminopimelate and α-aminoadipate pathways, which employ distinct e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − form under biological conditions), and a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. It can be synthesized in the human body under normal physiological circumstances, making it a nonessential amino acid. It is encoded by the codons UCU, UCC, UCA, UCG, AGU and AGC. Occurrence This compound is one of the naturally occurring proteinogenic amino acids. Only the L-stereoisomer appears naturally in proteins. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine. Serine was first obtained from silk protein, a particularly rich source, in 1865 by Emil Cramer. Its name is derived from the Latin for silk, ''sericum''. Serine's structure was estab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoleucine
Isoleucine (symbol Ile or I) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO form under biological conditions), and a hydrocarbon side chain with a branch (a central carbon atom bound to three other carbon atoms). It is classified as a non-polar, uncharged (at physiological pH), branched-chain, aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it, and must be ingested in our diet. Isoleucine is synthesized from pyruvate employing leucine biosynthesis enzymes in other organisms such as bacteria. It is encoded by the codons AUU, AUC, and AUA. Metabolism Biosynthesis As an essential nutrient, it is not synthesized in the body, hence it must be ingested, usually as a component of proteins. In plants and microorganisms, it is synthesized via several steps, startin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leucine
Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α- carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain isobutyl group, making it a non-polar aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Human dietary sources are foods that contain protein, such as meats, dairy products, soy products, and beans and other legumes. It is encoded by the codons UUA, UUG, CUU, CUC, CUA, and CUG. Like valine and isoleucine, leucine is a branched-chain amino acid. The primary metabolic end products of leucine metabolism are acetyl-CoA and acetoacetate; consequently, it is one of the two exclusively ketogenic amino acids, with lysine being the other. It is the most import ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

-3D-balls.png)