|
Perkow Reaction
The Perkow reaction is an organic reaction in which a trialkyl phosphite ester reacts with a haloketone to form a dialkyl vinyl phosphate and an alkyl halide. In the related Michaelis–Arbuzov reaction the same reactants are known to form a beta-keto phosphonate which is an important reagent in the Horner–Wadsworth–Emmons reaction on the road to alkenes. The Perkow reaction, in this respect is considered a side-reaction. Reaction mechanism The reaction mechanism of the Perkow reaction consists of a nucleophilic addition of the phosphite at the carbonyl carbon forming a zwitterionic intermediate. The zwitterionic intermediate rearranges to a cationic species while eliminating the halide. The cationic species then dealkylates through a second nucleophilic displacement in which the halide anion attacks one of the phosphite alkoxide substituents forming an enol phosphate.Organophosphorus chemistry. XVII. Kinetics and mechanism of the Perkow reaction'' Irving J. Borowitz , St ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, Mechanistic Organic Photochemistry, photochemical reactions and organic redox reaction, redox reactions. In organic synthesis, organic reactions are used in the construction of new organic molecules. The production of many man-made chemicals such as drugs, plastics, food additives, fabrics depend on organic reactions. The oldest organic reactions are combustion of organic fuels and saponification of fats to make soap. Modern organic chemistry starts with the Wöhler synthesis in 1828. In the history of the Nobel Prize in Chemistry awards have been given for the invention of specific organic reactions such as the Grignard reaction in 1912, the Diels-Alder reaction in 1950, the Wittig reaction in 1979 and olefin metathesis in 2005. Classifications Organic c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triethylphosphite
Triethyl phosphite is an organophosphorus compound with the formula P(OCH2CH3)3, often abbreviated P(OEt)3. It is a colorless, malodorous liquid. It is used as a ligand in organometallic chemistry and as a reagent in organic synthesis The molecule features a pyramidal phosphorus(III) center bound to three ethoxide groups. Its 31P NMR spectrum features a signal at around +139 ppm vs phosphoric acid standard. Triethylphosphite is prepared by treating phosphorus trichloride with ethanol in the presence of a base, typically a tertiary amine: :PCl3 + 3 EtOH + 3 R3N → P(OEt)3 + 3 R3NH + Cl− In the absence of the base, the reaction affords diethylphosphite ((EtO)2P(O)H). Of the many related compounds can be prepared similarly, triisopropyl phosphite is an example (b.p. 43.5 °C/1.0 mm; CAS# 116-17-6). As a ligand In coordination chemistry and homogeneous catalysis, triethylphosphite finds use as a soft ligand. Its complexes are generally lipophilic and feature met ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine Monophosphate
Adenosine monophosphate (AMP), also known as 5'-adenylic acid, is a nucleotide. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine; it is an ester of phosphoric acid and the nucleoside adenosine. As a substituent it takes the form of the prefix adenylyl-. AMP plays an important role in many cellular metabolic processes, being interconverted to Adenosine diphosphate, ADP and/or Adenosine triphosphate, ATP. AMP is also a component in the synthesis of RNA. AMP is present in all known forms of life. Production and degradation AMP does not have the high energy phosphoanhydride bond associated with ADP and ATP. AMP can be produced from Adenosine diphosphate, ADP: : 2 ADP → ATP + AMP Or AMP may be produced by the hydrolysis of one high energy phosphate bond of ADP: : ADP + H2O → AMP + phosphate, Pi AMP can also be formed by hydrolysis of Adenosine triphosphate, ATP into AMP and pyrophosphate: : ATP + H2O → AMP + pyrophosphate, PPi When RNA i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorylation
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. Protein phosphorylation often activates (or deactivates) many enzymes. Glucose Phosphorylation of sugars is often the first stage in their catabolism. Phosphorylation allows cells to accumulate sugars because the phosphate group prevents the molecules from diffusing back across their transporter. Phosphorylation of glucose is a key reaction in sugar metabolism. The chemical equation for the conversion of D-glucose to D-glucose-6-phosphate in the first step of glycolysis is given by :D-glucose + ATP → D-glucose-6-phosphate + ADP : ΔG° = −16.7 kJ/mol (° indicates measurement at standard condition) Hepatic cells are freely permeable to glucose, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enol
In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene ( olefin) with a hydroxyl group attached to one end of the alkene double bond (). The terms ''enol'' and ''alkenol'' are portmanteaus deriving from "-ene"/"alkene" and the "-ol" suffix indicating the hydroxyl group of alcohols, dropping the terminal "-e" of the first term. Generation of enols often involves removal of a hydrogen adjacent (α-) to the carbonyl group—i.e., deprotonation, its removal as a proton, . When this proton is not returned at the end of the stepwise process, the result is an anion termed an enolate (see images at right). The enolate structures shown are schematic; a more modern representation considers the molecular orbitals that are formed and occupied by electrons in the enolate. Similarly, generation of the enol often is accompanied by "trapping" or masking of the hydroxy group as an ether, such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enol Ether
In organic chemistry an enol ether is an alkene with an alkoxy substituent. The general structure is R2C=CR-OR where R = H, alkyl or aryl. A common subfamily of enol ethers are vinyl ethers, with the formula ROCH=CH2. Important enol ethers include the reagent 3,4-dihydropyran and the monomers methyl vinyl ether and ethyl vinyl ether. Reactions and uses Akin to enamines, enol ethers are electron-rich alkenes by virtue of the electron-donation from the heteroatom via pi-bonding. Enol ethers have oxonium ion character. By virtue of their bonding situation, enol ethers display distinctive reactivity. In comparison with simple alkenes, enol ethers exhibit enhanced susceptibility to attack by electrophiles such as Bronsted acids. Similarly, they undergo inverse demand Diels-Alder reactions. The reactivity of enol ethers is highly dependent on the presence of substituents alpha to oxygen. The vinyl ethers are susceptible to polymerization to give polyvinyl ethers. Some vinyl ethers al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, which may be replaced by some other element or compound to serve as a functional group. Phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring. Nomenclature Usually, a "phenyl group" is synonymous with C6H5− and is represented by the symbol Ph or, archaically, Φ. Benzene is sometimes denoted as PhH. Phenyl groups are generally attached to other atoms or groups. For example, triphenylmethane (Ph3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
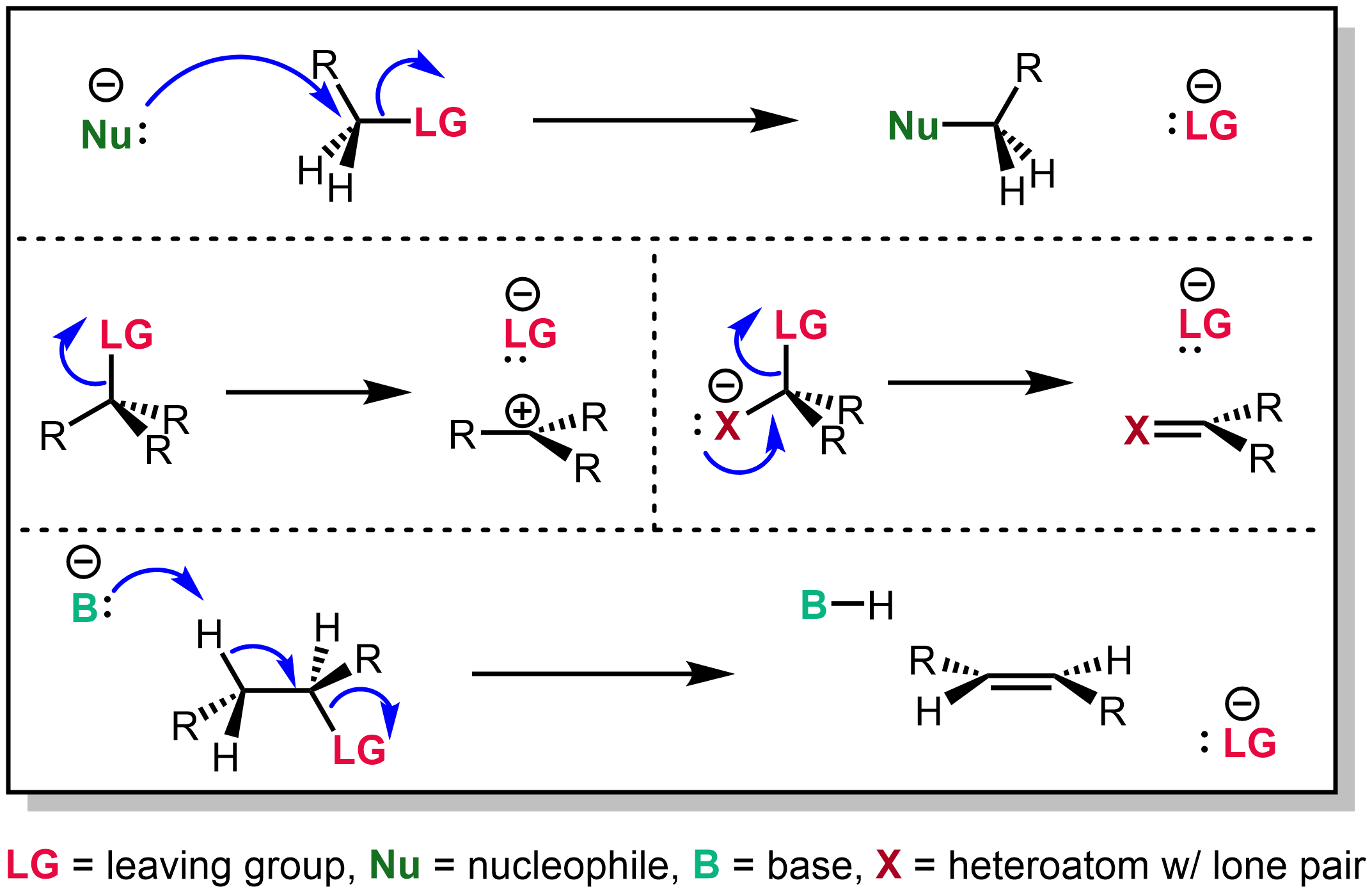
Leaving Group
In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited to a fragment that departs with a pair of electrons in heterolytic bond cleavage. In this usage, a leaving group is a less formal but more commonly used synonym of the term '' nucleofuge''. In this context, leaving groups are generally anions or neutral species, departing from a neutral or cationic substrates, respectively, though in rare cases, cations leaving from a dicationic substrate are also known. A species' ability to serve as a leaving group depends on its ability to stabilize the additional electron density that results from bond heterolysis. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters such as tosylate (TsO−), while water (H2O), alcohols (HOR), and amines (R3N) are common neutr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane. The isomer ''n''-butane can connect in two ways, giving rise to two "-butyl" groups: * If it connects at one of the two terminal carbon atoms, it is normal butyl or ''n''-butyl: (preferred IUPAC name: butyl) * If it connects at one of the non-terminal (internal) carbon atoms, it is secondary butyl or ''sec''-butyl: (preferred IUPAC name: butan-2-yl) The second isomer of butane, isobutane, can also connect in two ways, giving rise to two additional groups: * If it connects at one of the three terminal carbons, it is isobutyl: (preferred IUPAC name: 2-methylpropyl) * If it connects at the central carbon, it is tertiary butyl, ''tert''-butyl or ''t''-butyl: (preferred IUPAC name: ''tert''-butyl) Nomenclature According to IUPAC nomenclature, "isobutyl", "''sec''-butyl", and "''tert''-b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinoline
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. Over 200 biologically active quinoline and quinazoline alkaloids are identified. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance. Occurrence and isolation Quinoline was first extracted from coal tar in 1834 by German chemist Friedlieb Ferdinand Runge; he called quinoline ''leukol'' ("white oil" in Greek). Coal tar remains the principal source of commercial quinoline. In 1842, French chemist Charles Gerhardt obtained a compound by dry distilling quinine, stry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |