|
Oxyride Battery
''Nickel oxyhydroxide battery'' (abbr. NiOx, IEC code: Z) is a type of primary cell. It is not rechargeable and must be disposed after a single use. NiOx batteries can be used in high-drain applications such as digital cameras. NiOx batteries used in low-drain applications, have a lifespan similar to an alkaline battery. NiOx batteries produce a higher voltage (1.7V) than alkaline batteries (1.5V) which can cause problems in certain products, such as equipment with incandescent light bulbs (such as flashlights/torches), or devices without a voltage regulator. Construction The nickel oxyhydroxide cell is different from a standard alkaline battery in the manufacturing process and in chemical composition. The chemical difference is the addition of nickel oxyhydroxide to the manganese dioxide and graphite for the cathode. This results in an unloaded voltage of 1.7 V DC per cell. The cells sustain a higher average voltage during discharge compared to alkaline batteries. This may ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primary Cell
A primary battery or primary cell is a battery (a galvanic cell) that is designed to be used once and discarded, and not recharged with electricity and reused like a secondary cell (rechargeable battery). In general, the electrochemical reaction occurring in the cell is not reversible, rendering the cell unrechargeable. As a primary cell is used, chemical reactions in the battery use up the chemicals that generate the power; when they are gone, the battery stops producing electricity. In contrast, in a secondary cell, the reaction can be reversed by running a current into the cell with a battery charger to recharge it, regenerating the chemical reactants. Primary cells are made in a range of standard sizes to power small household appliances such as flashlights and portable radios. Primary batteries make up about 90% of the $50 billion battery market, but secondary batteries have been gaining market share. About 15 billion primary batteries are thrown away worldwide every year, vi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Digital Camera
A digital camera is a camera that captures photographs in digital memory. Most cameras produced today are digital, largely replacing those that capture images on photographic film. Digital cameras are now widely incorporated into mobile devices like smartphones with the same or more capabilities and features of dedicated cameras (which are still available). High-end, high-definition dedicated cameras are still commonly used by professionals and those who desire to take higher-quality photographs. Digital and digital movie cameras share an optical system, typically using a lens with a variable diaphragm to focus light onto an image pickup device. The diaphragm and shutter admit a controlled amount of light to the image, just as with film, but the image pickup device is electronic rather than chemical. However, unlike film cameras, digital cameras can display images on a screen immediately after being recorded, and store and delete images from memory. Many digital cameras can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Incandescent Light Bulbs
An incandescent light bulb, incandescent lamp or incandescent light globe is an electric light with a wire filament heated until it glows. The filament is enclosed in a glass bulb with a vacuum or inert gas to protect the filament from oxidation. Current is supplied to the filament by terminals or wires embedded in the glass. A bulb socket provides mechanical support and electrical connections. Incandescent bulbs are manufactured in a wide range of sizes, light output, and voltage ratings, from 1.5 volts to about 300 volts. They require no external regulating equipment, have low manufacturing costs, and work equally well on either alternating current or direct current. As a result, the incandescent bulb became widely used in household and commercial lighting, for portable lighting such as table lamps, car headlamps, and flashlights, and for decorative and advertising lighting. Incandescent bulbs are much less efficient than other types of electric lighting, converting less ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaline Battery
An alkaline battery (IEC code: L) is a type of primary battery where the electrolyte (most commonly potassium hydroxide) has a pH value above 7. Typically these batteries derive energy from the reaction between zinc metal and manganese dioxide, nickel and cadmium, or nickel and hydrogen. Compared with zinc–carbon batteries of the Leclanché cell or zinc chloride types, alkaline batteries have a higher energy density and longer shelf life, yet provide the same voltage. The alkaline battery gets its name because it has an alkaline electrolyte of potassium hydroxide (KOH) instead of the acidic ammonium chloride (NH4Cl) or zinc chloride (ZnCl2) electrolyte of the zinc–carbon batteries. Other battery systems also use alkaline electrolytes, but they use different active materials for the electrodes. Alkaline batteries account for 80% of manufactured batteries in the US and over 10 billion individual units produced worldwide. In Japan, alkaline batteries account for 46% of all ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel Oxide Hydroxide
Nickel oxide hydroxide is the inorganic compound with the chemical formula NiO(OH). It is a black solid that is insoluble in all solvents but attacked by base and acid. It is a component of the nickel-metal hydride battery and of the nickel–iron battery. Related materials Nickel(III) oxides are often poorly characterized and are assumed to be nonstoichiometric compounds. Nickel(III) oxide (Ni2O3) has not been verified crystallographically. For applications in organic chemistry, nickel oxides or peroxides are generated in situ and lack crystallographic characterization. For example, "nickel peroxide" ( CAS# 12035-36-8) is also closely related to or even identical with NiO(OH). Synthesis and structure Its layered structure resembles that of the brucite polymorph of nickel(II) hydroxide, but with half as many hydrogens. The oxidation state of nickel is 3+. It can be prepared by the reaction of nickel(II) hydroxide with aqueous potassium hydroxide and bromine as the oxidant: : 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese Dioxide
Manganese dioxide is the inorganic compound with the formula . This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for is for dry-cell batteries, such as the alkaline battery and the zinc–carbon battery.. is also used as a pigment and as a precursor to other manganese compounds, such as . It is used as a reagent in organic synthesis, for example, for the oxidation of allylic alcohols. is α polymorph that can incorporate a variety of atoms (as well as water molecules) in the "tunnels" or "channels" between the manganese oxide octahedra. There is considerable interest in as a possible cathode for lithium-ion batteries. Structure Several polymorphs of are claimed, as well as a hydrated form. Like many other dioxides, crystallizes in the rutile crystal structure (this polymorph is called pyrolusite or ), with three-coordinate oxide and octahedral metal centres. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
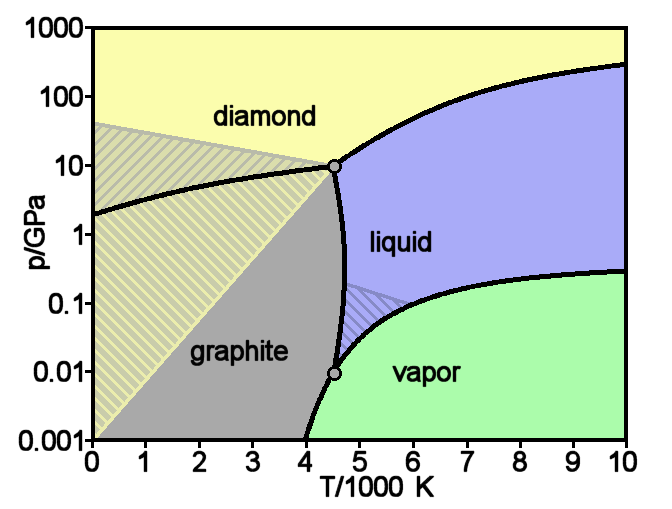
Graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on large scale (300 kton/year, in 1989) for uses in pencils, lubricants, and electrodes. Under high pressures and temperatures it converts to diamond. It is a weak conductor of heat and electricity. Types and varieties Natural graphite The principal types of natural graphite, each occurring in different types of ore deposits, are * Crystalline small flakes of graphite (or flake graphite) occurs as isolated, flat, plate-like particles with hexagonal edges if unbroken. When broken the edges can be irregular or angular; * Amorphous graphite: very fine flake graphite is sometimes called amorphous; * Lump graphite (or vein graphite) occurs in fissure veins or fractures and appears as massive platy intergrowths of fibrous or acicular crystalline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction in which positive charges move. Electrons have a negative electrical charge, so the movement of electrons is opposite to that of the conventional current flow. Consequently, the mnemonic ''cathode current departs'' also means that electrons flow ''into'' the device's cathode from the external circuit. For example, the end of a household battery marked with a + (plus) is the cathode. The electrode through which conventional current flows the other way, into the device, is termed an anode. Charge flow Conventional current flows from cathode to anode outside of the cell or device (with electrons moving in the opposite direction), regardless of the cell or device type and operating mode. Cathode polarity with respect to the anode can be positive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Battery Types ...
This list is a summary of notable electric battery types composed of one or more electrochemical cells. Three lists are provided in the table. The primary (non-rechargeable) and secondary (rechargeable) cell lists are lists of battery chemistry. The third list is a list of battery applications. Battery cell types See also * Baghdad Battery * Battery nomenclature * Carnot battery * Comparison of commercial battery types * History of the battery * List of battery sizes * List of energy densities * ''Search for the Super Battery'' (2017 PBS film) * Fuel cell References {{Battery sizes * Battery Battery most often refers to: * Electric battery, a device that provides electrical power * Battery (crime), a crime involving unlawful physical contact Battery may also refer to: Energy source *Automotive battery, a device to provide power t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
David Pogue
David Welch Pogue (born March 9, 1963) is an American technology and science writer and TV presenter. He is an Emmy-winning correspondent for ''CBS News Sunday Morning'' and author of the "Crowdwise" column in ''The New York Times'' Smarter Living section. He has hosted 18 ''Nova'' specials on PBS, including '' NOVA ScienceNow'', the ''Making Stuff'' series in 2011 and 2013, and ''Hunting the Elements'' in 2012. Pogue has written or co-written seven books in the ''For Dummies'' series (including Macintosh computers, magic, opera, and classical music). In 1999, he launched his own series of computer how-to books called the '' Missing Manual'' series, which now includes more than 100 titles covering a variety of Mac and Windows operating systems and applications. Among the dozens of books Pogue has authored is ''The World According to Twitter'' (2009), written in collaboration with around 500,000 of his Twitter followers, and ''Pogue's Basics'' (2014), which was a ''New York Time ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |