|
Oxford–AstraZeneca COVID-19 Vaccine
The Oxford–AstraZeneca COVID19 vaccine, sold under the brand names Covishield and Vaxzevria among others, is a viral vector vaccine for prevention of COVID-19. Developed in the United Kingdom by Oxford University and British-Swedish company AstraZeneca, using as a vector the modified chimpanzee adenovirus ChAdOx1. The vaccine is given by intramuscular injection. Studies carried out in 2020 showed that the efficacy of the vaccine is 76.0% at preventing symptomatic COVID-19 beginning at 22 days following the first dose, and 81.3% after the second dose. A study in Scotland found that, for symptomatic COVID-19 infection after the second dose, the vaccine is 81% effective against the Alpha variant (lineage B.1.1.7), and 61% against the Delta variant (lineage B.1.617.2). The vaccine is stable at refrigerator temperatures and has a good safety profile, with side effects including injection-site pain, headache, and nausea, all generally resolving within a few days. More rarely, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AstraZeneca
AstraZeneca plc () is a British-Swedish multinational pharmaceutical and biotechnology company with its headquarters at the Cambridge Biomedical Campus in Cambridge, England. It has a portfolio of products for major diseases in areas including oncology, cardiovascular, gastrointestinal, infection, neuroscience, respiratory, and inflammation. It has been involved in developing the Oxford–AstraZeneca COVID-19 vaccine. The company was founded in 1999 through the merger of the Swedish Astra AB and the British Zeneca Group (itself formed by the demerger of the pharmaceutical operations of Imperial Chemical Industries in 1993). Since the merger it has been among the world's largest pharmaceutical companies and has made numerous corporate acquisitions, including Cambridge Antibody Technology (in 2006), MedImmune (in 2007), Spirogen (in 2013) and Definiens (by MedImmune in 2014). It has its research and development concentrated in three strategic centres: Cambridge, England; ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EudraCT
EudraCT (European Union Drug Regulating Authorities Clinical Trials) is the European clinical trials registry, Clinical Trials Database of all clinical trials of investigational medicinal products with at least one site in the European Union commencing 1 May 2004 or later. The EudraCT database has been established in accordance with Directive 2001/20/EC. The EudraCT Number is unique and is needed on other documents relating to the trials (e.g. Serious adverse event, SUSAR reports). Public Side The public side of EudraCT is for organisations to register any of their clinical trials as defined by Directive 2001/20/EC. The process of applying and registering a clinical trial should be completed before submitting an application to any of the Member State/s in which they anticipate running the trial. The public side of EudraCT does not save any of the trial detail entered by the user, and instead provides a saved data file in the form of an XML which the user must store on their own loc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
World Health Organization
The World Health Organization (WHO) is a specialized agency of the United Nations responsible for international public health. The WHO Constitution states its main objective as "the attainment by all peoples of the highest possible level of health". Headquartered in Geneva, Switzerland, it has six regional offices and 150 field offices worldwide. The WHO was established on 7 April 1948. The first meeting of the World Health Assembly (WHA), the agency's governing body, took place on 24 July of that year. The WHO incorporated the assets, personnel, and duties of the League of Nations' Health Organization and the , including the International Classification of Diseases (ICD). Its work began in earnest in 1951 after a significant infusion of financial and technical resources. The WHO's mandate seeks and includes: working worldwide to promote health, keeping the world safe, and serve the vulnerable. It advocates that a billion more people should have: universal health care coverag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
COVID-19 Vaccination In The United Kingdom
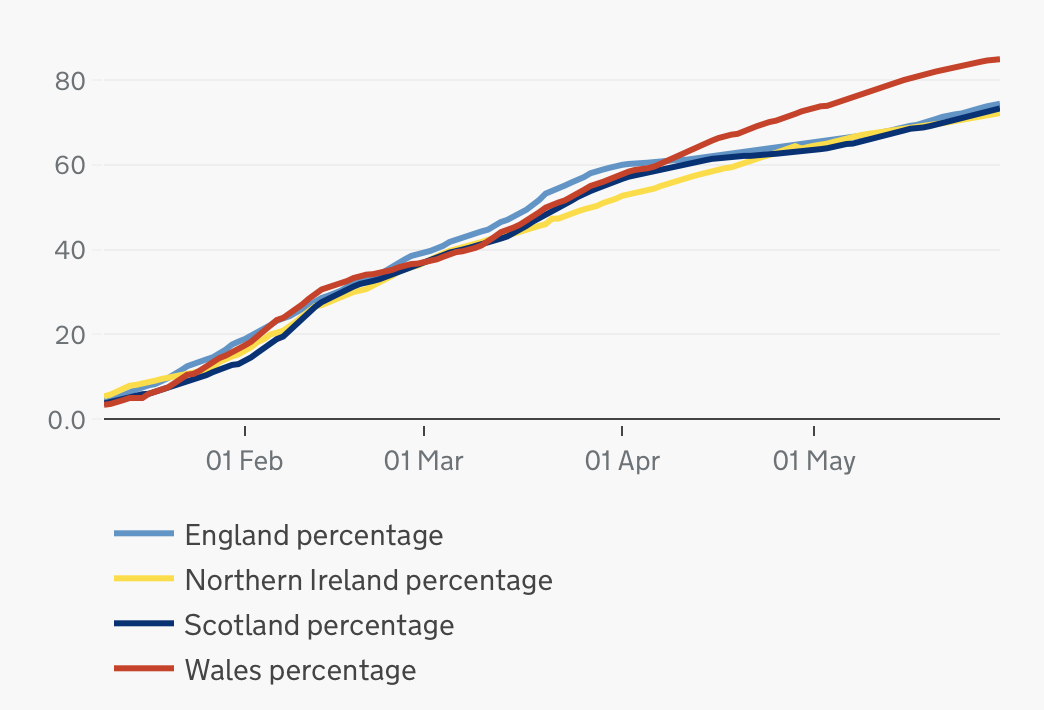
The COVID-19 vaccination programme in the United Kingdom is an ongoing mass immunisation campaign for coronavirus disease 2019 (COVID-19) during the COVID-19 pandemic in the United Kingdom. Vaccinations began on 8 December 2020 after Margaret Keenan became the first person in the world (outside trials) to receive her first dose of two of the Pfizer–BioNTech COVID-19 vaccine. There are three vaccines currently in use; following approval of the Pfizer–BioNTech COVID-19 vaccine ( Comirnaty), vaccines developed by University of Oxford and AstraZeneca ( Vaxzevria), and the United States National Institute of Allergy and Infectious Diseases and Moderna (Spikevax) have been rolled out. , there were four other COVID-19 vaccines on order for the programme, at varying stages of development. Phase 1 of the rollout prioritised the most vulnerable, in a schedule primarily based on age. The delivery plan was adjusted on 30 December 2020, delaying second doses so that more people cou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Economic Area
The European Economic Area (EEA) was established via the ''Agreement on the European Economic Area'', an international agreement which enables the extension of the European Union's single market to member states of the European Free Trade Association. The EEA links the EU member states and three EFTA states (Iceland, Liechtenstein, and Norway) into an internal market governed by the same basic rules. These rules aim to enable free movement of persons, goods, services, and capital within the European single market, including the freedom to choose residence in any country within this area. The EEA was established on 1 January 1994 upon entry into force of the EEA Agreement. The contracting parties are the EU, its member states, and Iceland, Liechtenstein, and Norway. The EEA Treaty is a commercial treaty and differs from the EU Treaties in certain key respects. According to Article 1 its purpose is to "promote a continuous and balanced strengthening of trade and economic relati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Embolic And Thrombotic Events After COVID-19 Vaccination
Post-vaccination embolic and thrombotic events, termed vaccine-induced immune thrombotic thrombocytopenia (VITT), vaccine-induced prothrombotic immune thrombocytopenia (VIPIT), thrombosis with thrombocytopenia syndrome (TTS), vaccine-induced immune thrombocytopenia and thrombosis (VITT), or vaccine-associated thrombotic thrombocytopenia (VATT), are rare types of blood clotting syndromes that were initially observed in a number of people who had previously received the Oxford–AstraZeneca COVID‑19 vaccine (AZD1222) during the COVID‑19 pandemic. It was subsequently also described in the Janssen COVID‑19 vaccine (Johnson & Johnson) leading to suspension of its use until its safety had been reassessed. On 5 May 2022 the FDA posted a bulletin limiting the use of the Janssen Vaccine to very specific cases due to further reassesment of the risks of TTS, although the FDA also stated in the same bulletin that the benefits of the vaccine outweigh the risks. In April 2021, AstraZen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thrombocytopenia
Thrombocytopenia is a condition characterized by abnormally low levels of platelets, also known as thrombocytes, in the blood. It is the most common coagulation disorder among intensive care patients and is seen in a fifth of medical patients and a third of surgical patients. A normal human platelet count ranges from 150,000 to 450,000 platelets/microliter (μl) of blood. Values outside this range do not necessarily indicate disease. One common definition of thrombocytopenia requiring emergency treatment is a platelet count below 50,000/μl. Thrombocytopenia can be contrasted with the conditions associated with an abnormally ''high'' level of platelets in the blood - thrombocythemia (when the cause is unknown), and thrombocytosis (when the cause is known). Signs and symptoms Thrombocytopenia usually has no symptoms and is picked up on a routine complete blood count. Some individuals with thrombocytopenia may experience external bleeding, such as nosebleeds or bleeding gums. S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anaphylaxis
Anaphylaxis is a serious, potentially fatal allergic reaction and medical emergency that is rapid in onset and requires immediate medical attention regardless of use of emergency medication on site. It typically causes more than one of the following: an itchy rash, throat closing due to swelling which can obstruct or stop breathing; severe tongue swelling which can also interfere with or stop breathing; shortness of breath, vomiting, lightheadedness, loss of consciousness, low blood pressure, and medical shock. These symptoms typically start in minutes to hours and then increase very rapidly to life-threatening levels. Urgent medical treatment is required to prevent serious harm or death, even if the patient has used an epipen or has taken other medications in response, and even if symptoms appear to be improving. Common causes include allergies to insect bites and stings, allergies to foods – including nuts, milk, fish, shellfish, eggs and some fresh fruits or dried fruits; a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
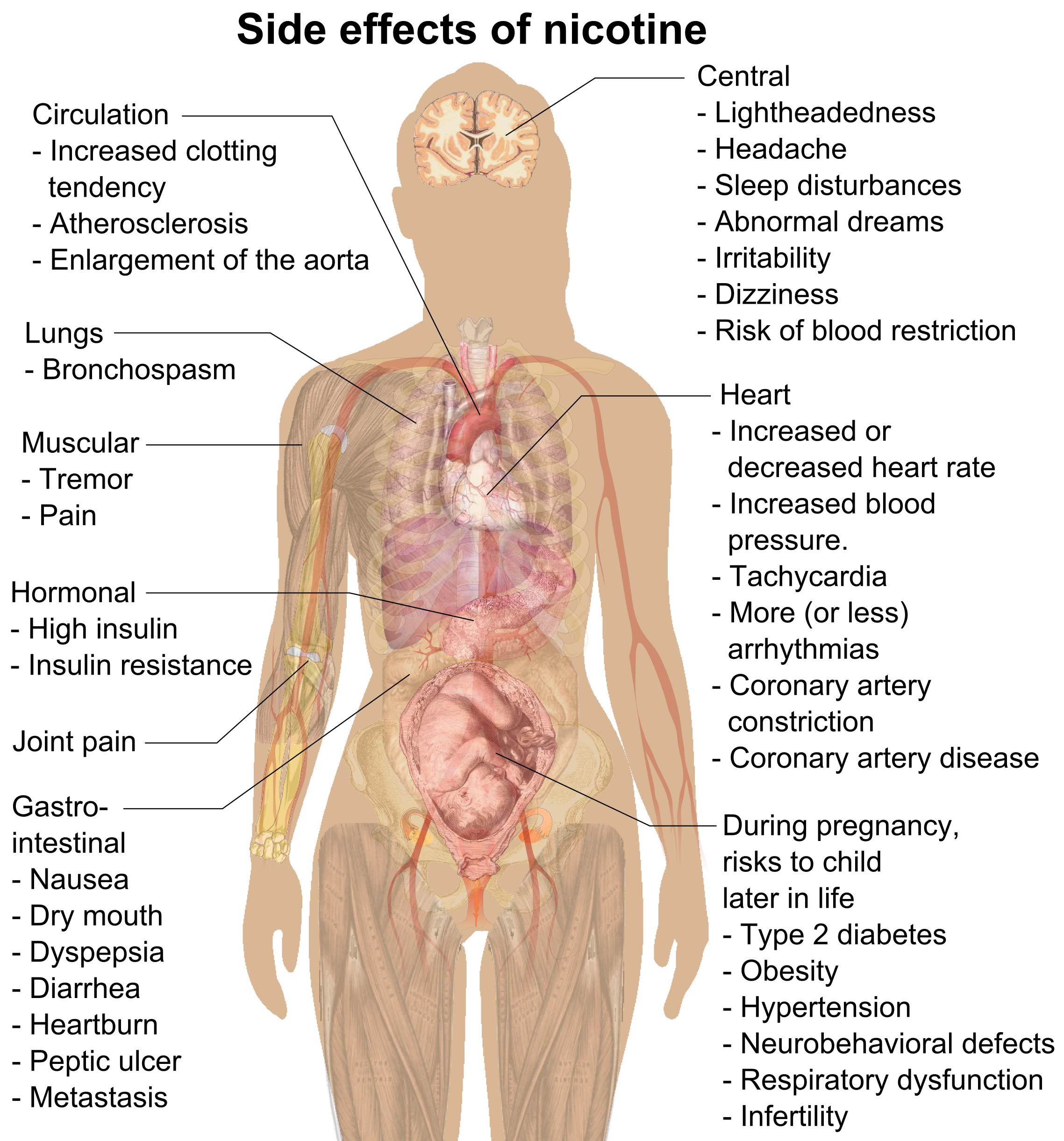
Side Effect
In medicine, a side effect is an effect, whether therapeutic or adverse, that is secondary to the one intended; although the term is predominantly employed to describe adverse effects, it can also apply to beneficial, but unintended, consequences of the use of a drug. Developing drugs is a complicated process, because no two people are exactly the same, so even drugs that have virtually no side effects, might be difficult for some people. Also, it is difficult to make a drug that targets one part of the body but that does not affect other parts, the fact that increases the risk of side effects in the untargeted parts. Occasionally, drugs are prescribed or procedures performed specifically for their side effects; in that case, said side effect ceases to be a side effect and is now an intended effect. For instance, X-rays were historically (and are currently) used as an imaging technique; the discovery of their oncolytic capability led to their employ in radiotherapy (ablation o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SARS-CoV-2 Delta Variant
The Delta variant (B.1.617.2) was a variant of SARS-CoV-2, the virus that causes COVID-19. It was first detected in India in late 2020. The Delta variant was named on 31 May 2021 and had spread to over 179 countries by 22 November 2021. The World Health Organization (WHO) indicated in June 2021 that the Delta variant was becoming the dominant strain globally. It has mutations in the gene encoding the SARS-CoV-2 coronavirus spike protein, spike protein causing the substitutions T478K, Variants of SARS-CoV-2#P681R, P681R and Variants of SARS-CoV-2#L452R, L452R, which are known to affect transmissibility of the virus as well as whether it can be neutralised by antibodies for previously circulating variants of the COVID-19 virus. In August 2021, Public Health England (PHE) reported secondary attack rate in household contacts of non-travel or unknown cases for Delta to be 10.8% ''vis-à-vis'' 10.2% for the SARS-CoV-2 Alpha variant, Alpha variant; the case fatality rate for those ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SARS-CoV-2 Alpha Variant
The Alpha variant (B.1.1.7) was a Variants of SARS-CoV-2, SARS-CoV-2 variant of concern. It was estimated to be 40–80% more Transmission (medicine), transmissible than the Wild type, wild-type SARS-CoV-2 (with most estimates occupying the middle to higher end of this range). It was first detected in November 2020 from a sample taken in September in the United Kingdom, and began to spread quickly by mid-December, around the same time as infections surged. This increase is thought to be at least partly because of one or more mutations in the virus' coronavirus spike protein, spike protein. The variant was also notable for having more mutations than normally seen. By January 2021, more than half of all genomic sequencing of SARS-CoV-2 was carried out in the UK. This had given rise to questions as to how many other important variants were circulating around the world undetected. On 2 February 2021, Public Health England reported that they had detected "[a] limited number of B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vaccine Efficacy
Vaccine efficacy or vaccine effectiveness is the percentage reduction of disease cases in a vaccinated group of people compared to an unvaccinated group. For example, a vaccine efficacy or effectiveness of 80% indicates an 80% decrease in the number of disease cases among a group of vaccinated people compared to a group in which nobody was vaccinated. When a study is carried out using the most favorable, ideal or perfectly controlled conditions, such as those in a clinical trial, the term ''vaccine efficacy'' is used. On the other hand, when a study is carried out to show how well a vaccine works when they are used in a bigger, typical population under less-than-perfectly controlled conditions, the term ''vaccine effectiveness'' is used. Vaccine efficacy was designed and calculated by Greenwood and Yule in 1915 for the cholera and typhoid vaccines. It is best measured using double-blind, randomized, clinical controlled trials, such that it is studied under "best case scenario ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_2021_C.jpg)
_I_(cropped).jpg)