|
Overtone Band
In vibrational spectroscopy, an overtone band is the spectral band that occurs in a vibrational spectrum of a molecule when the molecule makes a transition from the ground state (v=0) to the second excited state (v=2), where v is the vibrational quantum number (a non-negative integer) obtained from solving the Schrödinger equation for the molecule. Generally, in order to study the vibrational spectra of molecules, chemical bond vibrations are assumed to be approximable as simple harmonic oscillators. Thus a quadratic potential is used in the Schrödinger equation to solve for the vibrational energy eigenstates and their eigenvalues. These energy states are quantized, meaning they can assume only some "discrete" values of energy. When electromagnetic radiation is shined on a sample, the molecules can absorb energy from the radiation and change their vibrational energy state. However, the molecules can absorb energy from radiation only under certain condition, namely- there should b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vibrational Spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms. It can be used to characterize new materials or identify and verify known and unknown samples. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer (or spectrophotometer) which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance (or transmittance) on the vertical axis vs. frequency, wavenumber or wavelength on the horizontal axis. Typical units of wavenumber used in IR spectra are reciprocal centimeters, with the symbol cm−1. Units of IR wavelength are commonly given in micrometers (formerly called "microns"), symbol μm, which are related to the wavenumber in a reciprocal wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selection Rule
In physics and chemistry, a selection rule, or transition rule, formally constrains the possible transitions of a system from one quantum state to another. Selection rules have been derived for electromagnetic transitions in molecules, in atoms, in atomic nuclei, and so on. The selection rules may differ according to the technique used to observe the transition. The selection rule also plays a role in chemical reactions, where some are formally spin-forbidden reactions, that is, reactions where the spin state changes at least once from reactants to products. In the following, mainly atomic and molecular transitions are considered. Overview In quantum mechanics the basis for a spectroscopic selection rule is the value of the ''transition moment integral'' :\int \psi_1^* \, \mu \, \psi_2 \, \mathrm\tau\,, where \psi_1 and \psi_2 are the wave functions of the two states, "state 1" and "state 2", involved in the transition, and is the transition moment operator. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Near-infrared Spectroscopy
Near-infrared spectroscopy (NIRS) is a spectroscopic method that uses the near-infrared region of the electromagnetic spectrum (from 780 nm to 2500 nm). Typical applications include medical and physiological diagnostics and research including blood sugar, pulse oximetry, functional neuroimaging, sports medicine, elite sports training, ergonomics, rehabilitation, neonatal research, brain computer interface, urology (bladder contraction), and neurology (neurovascular coupling). There are also applications in other areas as well such as pharmaceutical, food and agrochemical quality control, atmospheric chemistry, combustion research and astronomy. Theory Near-infrared spectroscopy is based on molecular overtone and combination vibrations. Such transitions are forbidden by the selection rules of quantum mechanics. As a result, the molar absorptivity in the near-IR region is typically quite small. (NIR absorption bands are typically 10–100 times weaker than the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fundamental Band
Fundamental may refer to: * Foundation of reality * Fundamental frequency, as in music or phonetics, often referred to as simply a "fundamental" * Fundamentalism, the belief in, and usually the strict adherence to, the simple or "fundamental" ideas based on faith in a system of thought * '' The Fundamentals'', a set of books important to Christian fundamentalism * Any of a number of fundamental theorems identified in mathematics, such as: ** Fundamental theorem of algebra, awe theorem regarding the factorization of polynomials ** Fundamental theorem of arithmetic, a theorem regarding prime factorization * Fundamental analysis, the process of reviewing and analyzing a company's financial statements to make better economic decisions Music * Fun-Da-Mental, a rap group * ''Fundamental'' (Bonnie Raitt album), 1998 * ''Fundamental'' (Pet Shop Boys album) * ''Fundamental'' (Puya album), 1999 * ''Fundamental'' (Mental As Anything album) * ''The Fundamentals'' (album) Other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intensity (physics)
In physics, the intensity or flux of radiant energy is the power transferred per unit area, where the area is measured on the plane perpendicular to the direction of propagation of the energy. In the SI system, it has units watts per square metre (W/m2), or kg⋅ s−3 in base units. Intensity is used most frequently with waves such as acoustic waves (sound) or electromagnetic waves such as light or radio waves, in which case the ''average'' power transfer over one period of the wave is used. ''Intensity'' can be applied to other circumstances where energy is transferred. For example, one could calculate the intensity of the kinetic energy carried by drops of water from a garden sprinkler. The word "intensity" as used here is not synonymous with " strength", "amplitude", " magnitude", or " level", as it sometimes is in colloquial speech. Intensity can be found by taking the energy density (energy per unit volume) at a point in space and multiplying it by the velocity at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IR Spectrum
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms. It can be used to characterize new materials or identify and verify known and unknown samples. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer (or spectrophotometer) which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance (or transmittance) on the vertical axis vs. frequency, wavenumber or wavelength on the horizontal axis. Typical units of wavenumber used in IR spectra are reciprocal centimeters, with the symbol cm−1. Units of IR wavelength are commonly given in micrometers (formerly called "microns"), symbol μm, which are related to the wavenumber in a reciprocal way. A c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
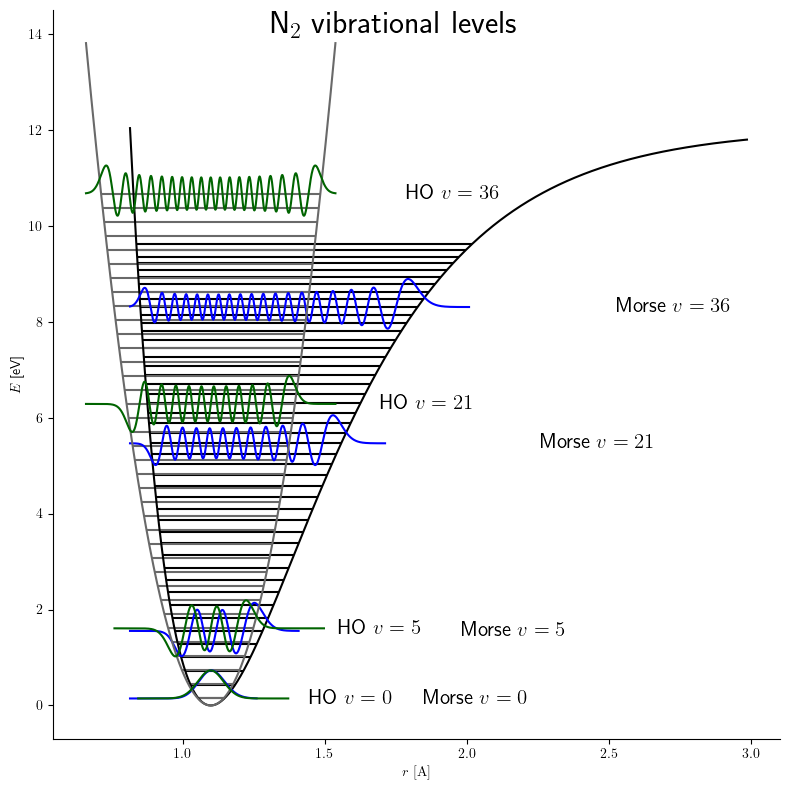
Morse Potential
The Morse potential, named after physicist Philip M. Morse, is a convenient interatomic interaction model for the potential energy of a diatomic molecule. It is a better approximation for the vibrational structure of the molecule than the quantum harmonic oscillator because it explicitly includes the effects of bond breaking, such as the existence of unbound states. It also accounts for the anharmonicity of real bonds and the non-zero transition probability for overtone and combination bands. The Morse potential can also be used to model other interactions such as the interaction between an atom and a surface. Due to its simplicity (only three fitting parameters), it is not used in modern spectroscopy. However, its mathematical form inspired the MLR ( Morse/Long-range) potential, which is the most popular potential energy function used for fitting spectroscopic data. Potential energy function The Morse potential energy function is of the form :V(r) = D_e ( 1-e^ )^2 Here ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Harmonic Oscillator
量子調和振動子 は、調和振動子, 古典調和振動子 の 量子力学, 量子力学 類似物です。任意の滑らかな ポテンシャル エネルギー, ポテンシャル は通常、安定した 平衡点 の近くで 調和振動子#単純調和振動子, 調和ポテンシャル として近似できるため、最も量子力学における重要なモデル系。さらに、これは正確な量子力学システムのリスト, 解析解法が知られている数少ない量子力学系の1つである。 author=Griffiths, David J. , title=量子力学入門 , エディション=2nd , 出版社=プレンティス・ホール , 年=2004 , isbn=978-0-13-805326-0 , author-link=David Griffiths (物理学者) , URL アクセス = 登録 , url=https://archive.org/details/introductiontoel00grif_0 One-dimensional harmonic oscillator Hamiltonian and energy eigenstates 粒子の ハミルトニアン (量子力学), ハミルトニアン は次の� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Dipole Moment
The transition dipole moment or transition moment, usually denoted \mathbf_ for a transition between an initial state, m, and a final state, n, is the electric dipole moment associated with the transition between the two states. In general the transition dipole moment is a complex vector quantity that includes the phase factors associated with the two states. Its direction gives the polarization of the transition, which determines how the system will interact with an electromagnetic wave of a given polarization, while the square of the magnitude gives the strength of the interaction due to the distribution of charge within the system. The SI unit of the transition dipole moment is the Coulomb-meter (Cm); a more conveniently sized unit is the Debye (D). Definition A single charged particle For a transition where a single charged particle changes state from , \psi_a \rangle to , \psi_b \rangle , the transition dipole moment \text is (\text a \rightarrow b) = \langle \ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ground State
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. In quantum field theory, the ground state is usually called the vacuum state or the vacuum. If more than one ground state exists, they are said to be degenerate. Many systems have degenerate ground states. Degeneracy occurs whenever there exists a unitary operator that acts non-trivially on a ground state and commutes with the Hamiltonian of the system. According to the third law of thermodynamics, a system at absolute zero temperature exists in its ground state; thus, its entropy is determined by the degeneracy of the ground state. Many systems, such as a perfect crystal lattice, have a unique ground state and therefore have zero entropy at absolute zero. It is also possible for the highest excited state to have absolute zero te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Dipole Moment
The electric dipole moment is a measure of the separation of positive and negative electrical charges within a system, that is, a measure of the system's overall polarity. The SI unit for electric dipole moment is the coulomb-meter (C⋅m). The debye (D) is another unit of measurement used in atomic physics and chemistry. Theoretically, an electric dipole is defined by the first-order term of the multipole expansion; it consists of two equal and opposite charges that are infinitesimally close together, although real dipoles have separated charge.Many theorists predict elementary particles can have very tiny electric dipole moments, possibly without separated charge. Such large dipoles make no difference to everyday physics, and have not yet been observed. (See electron electric dipole moment). However, when making measurements at a distance much larger than the charge separation, the dipole gives a good approximation of the actual electric field. The dipole is represented by a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |