|
Opacity Family Porins
Opacity family porins are a family of porin (protein), porins from pathogenic ''Neisseria''. These bacteria possess a repertoire of phase-variable opacity proteins that mediate various pathogen/host cell interactions. These proteins are related to OmpA-like transmembrane domain family. References Protein domains Protein families Outer membrane proteins {{membrane-protein-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Porin (protein)
Porins are beta barrel proteins that cross a cellular membrane and act as a pore, through which molecules can diffuse. Unlike other membrane transport proteins, porins are large enough to allow passive diffusion, i.e., they act as channels that are specific to different types of molecules. They are present in the outer membrane of gram-negative bacteria and some gram-positive mycobacteria (mycolic acid-containing actinomycetes), the outer membrane of mitochondria, and the outer chloroplast membrane (outer plastid membrane). Structure Porins are composed of beta sheets (β sheets) made up of beta strands (β strands) which are linked together by beta turns on the cytoplasmic side and long loops of amino acids on the other. The β strands lie in an antiparallel fashion and form a cylindrical tube, called a beta barrel (β barrel). The amino acid composition of the porin β strands are unique in that polar and nonpolar residues alternate along them. This means that the nonpolar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
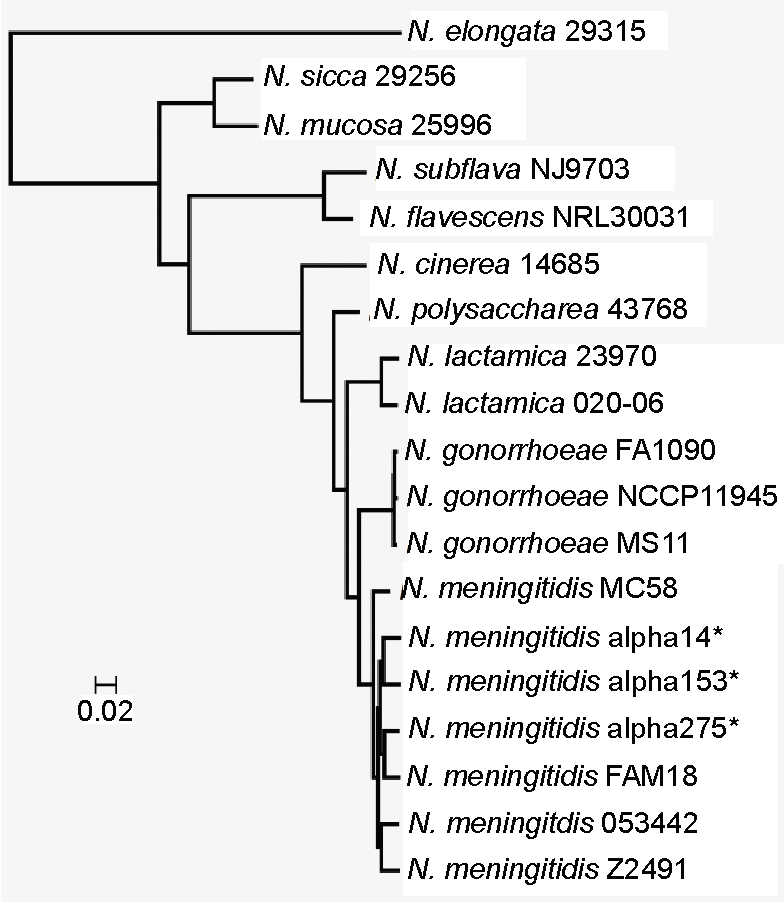
Neisseria
''Neisseria'' is a large genus of bacteria that colonize the mucosal surfaces of many animals. Of the 11 species that colonize humans, only two are pathogens, '' N. meningitidis'' and ''N. gonorrhoeae''. ''Neisseria'' species are Gram-negative bacteria included among the Pseudomonadota, a large group of Gram-negative forms. ''Neisseria'' diplococci resemble coffee beans when viewed microscopically. Pathogenesis and classification Pathogens Species of this genus (family Neisseriaceae) of parasitic bacteria grow in pairs and occasionally tetrads, and thrive best at 98.6 °F (37 °C) in the animal body or serum media. The genus includes: * ''N. gonorrhoeae'' (also called the gonococcus) causes gonorrhea. * '' N. meningitidis'' (also called the meningococcus) is one of the most common causes of bacterial meningitis and the causative agent of meningococcal septicaemia. These two species have the ability of 'breaching' the barrier. Local cytokines of the area become secr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
OmpA-like Transmembrane Domain
OmpA-like transmembrane domain is an evolutionarily conserved domain of bacterial outer membrane proteins. This domain consists of an eight-stranded beta barrel. OmpA is the predominant cell surface antigen in enterobacteria found in about 100,000 copies per cell. The expression of OmpA is tightly regulated by a variety of mechanisms. One mechanism by which OmpA expression is regulated in Vibrio species is by an antisense non-coding RNA called VrrA. Structure The structure consists of an eight-stranded Up-And-Down Beta-Barrel. The strands are connected by four extracellular loops and three intracellular turns. Function Numerous OmpA-like membrane-spanning domains contribute to bacterial virulence by a variety of mechanisms such as binding to host cells or immune regulators such as Factor H. Notable examples include ''E. coli'' OmpA and ''Yersinia pestis'' Ail. Several of these proteins are vaccine candidates. ''E. coli'' OmpA was shown to make specific interactions with t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domains
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufer after ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Families
A protein family is a group of evolutionarily related proteins. In many cases, a protein family has a corresponding gene family, in which each gene encodes a corresponding protein with a 1:1 relationship. The term "protein family" should not be confused with family as it is used in taxonomy. Proteins in a family descend from a common ancestor and typically have similar three-dimensional structures, functions, and significant sequence similarity. The most important of these is sequence similarity (usually amino-acid sequence), since it is the strictest indicator of homology and therefore the clearest indicator of common ancestry. A fairly well developed framework exists for evaluating the significance of similarity between a group of sequences using sequence alignment methods. Proteins that do not share a common ancestor are very unlikely to show statistically significant sequence similarity, making sequence alignment a powerful tool for identifying the members of protein familie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |