|
Orellanine Tautomerization
Orellanine or orellanin is a mycotoxin found in a group of mushrooms known as the Orellani of the family Cortinariaceae. Structurally, it is a bipyridine N-oxide compound somewhat related to the herbicide diquat. History Orellanine first came to people's attention in 1952 when a mass poisoning of 102 people in Konin, Poland, resulted in 11 deaths. Orellanine comes from a class of mushrooms that fall under the genus ''Cortinarius,'' and has been found in the species '' C. orellanus'', '' rubellus'', ''henrici'', '' rainerensis'' and '' bruneofulvus''. Poisonings related to these mushrooms have occurred predominately in Europe where mushroom foraging was common, though cases of orellanine poisoning have been reported in North America and Australia as well. There are several reported cases of people ingesting orellanine-containing mushrooms after mistaking them for edible or hallucinogenic mushrooms. Orellanine was first isolated in 1962, when Stanisław Grzymala extracted and iso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mycotoxin
A mycotoxin (from the Greek μύκης , "fungus" and τοξίνη , "toxin") is a toxic secondary metabolite produced by organisms of kingdom Fungi and is capable of causing disease and death in both humans and other animals. The term 'mycotoxin' is usually reserved for the toxic chemical products produced by fungi that readily colonize crops. Examples of mycotoxins causing human and animal illness include aflatoxin, citrinin, fumonisins, ochratoxin A, patulin, trichothecenes, zearalenone, and ergot alkaloids such as ergotamine. One mold species may produce many different mycotoxins, and several species may produce the same mycotoxin. Production Most fungi are aerobic (use oxygen) and are found almost everywhere in extremely small quantities due to the diminute size of their spores. They consume organic matter wherever humidity and temperature are sufficient. Where conditions are right, fungi proliferate into colonies and mycotoxin levels become high. The reason for the product ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridine-N-oxide
Pyridine-''N''-oxide is the heterocyclic compound with the formula C5H5NO. This colourless, hygroscopic solid is the product of the oxidation of pyridine. It was originally prepared using peroxyacids as the oxidising agent. The compound is used infrequently as an oxidizing reagent in organic synthesis. Structure The structure of pyridine-N-oxide is very similar to that of pyridine with respect to the parameters for the ring. The molecule is planar. The N-O distance is 1.34Å. The C-N-C angle is 124°, 7° wider than in pyridine. Synthesis The oxidation of pyridine can be achieved with a number of peracids including peracetic acid and perbenzoic acid. Oxidation can also be effected by a modified Dakin reaction using a urea-hydrogen peroxide complex, and sodium perborate or, using methylrhenium trioxide () as catalyst, with sodium percarbonate. Reactions Pyridine ''N''-oxide is five orders of magnitude less basic than pyridine, but it is isolable as a hydrochloride salt, 5 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine Triphosphatase
ATPases (, Adenosine 5'-TriPhosphatase, adenylpyrophosphatase, ATP monophosphatase, triphosphatase, SV40 T-antigen, ATP hydrolase, complex V (mitochondrial electron transport), (Ca2+ + Mg2+)-ATPase, HCO3−-ATPase, adenosine triphosphatase) are a class of enzymes that catalyze the decomposition of ATP into ADP and a free phosphate ion or the inverse reaction. This dephosphorylation reaction releases energy, which the enzyme (in most cases) harnesses to drive other chemical reactions that would not otherwise occur. This process is widely used in all known forms of life. Some such enzymes are integral membrane proteins (anchored within biological membranes), and move solutes across the membrane, typically against their concentration gradient. These are called transmembrane ATPases. Functions Transmembrane ATPases import metabolites necessary for cell metabolism and export toxins, wastes, and solutes that can hinder cellular processes. An important example is the sodium-pota ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
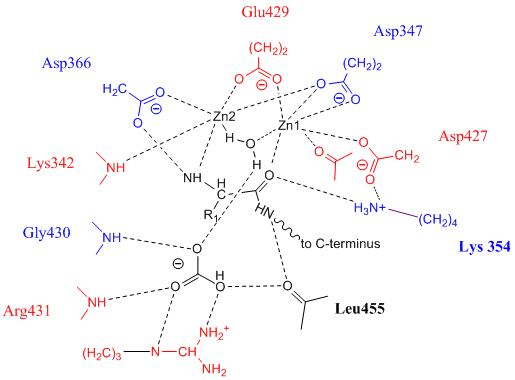
Leucyl Aminopeptidase
Leucyl aminopeptidases (, ''leucine aminopeptidase'', ''LAPs'', ''leucyl peptidase'', ''peptidase S'', ''cytosol aminopeptidase'', ''cathepsin III'', ''L-leucine aminopeptidase'', ''leucinaminopeptidase'', ''leucinamide aminopeptidase'', ''FTBL proteins'', ''proteinates FTBL'', ''aminopeptidase II'', ''aminopeptidase III'', ''aminopeptidase I'') are enzymes that preferentially catalyze the hydrolysis of leucine residues at the N-terminus of peptides and proteins. Other N-terminal residues can also be cleaved, however. LAPs have been found across superkingdoms. Identified LAPs include human LAP, bovine lens LAP, porcine LAP, ''Escherichia coli'' (''E. coli'') LAP (also known as PepA or XerB), and the solanaceous-specific acidic LAP (LAP-A) in tomato (''Solanum lycopersicum''). Enzyme description, structure, and active site The active sites in PepA and in bovine lens LAP have been found to be similar. Shown in the picture below is the proposed model for the active site of LAP-A in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gamma-glutamyltransferase
Gamma-glutamyltransferase (also γ-glutamyltransferase, GGT, gamma-GT, gamma-glutamyl transpeptidase; ) is a transferase (a type of enzyme) that catalyzes the transfer of gamma-glutamyl functional groups from molecules such as glutathione to an acceptor that may be an amino acid, a peptide or water (forming glutamate). GGT plays a key role in the gamma-glutamyl cycle, a pathway for the synthesis and degradation of glutathione as well as drug and xenobiotic detoxification. Other lines of evidence indicate that GGT can also exert a pro-oxidant role, with regulatory effects at various levels in cellular signal transduction and cellular pathophysiology. This transferase is found in many tissues, the most notable one being the liver, and has significance in medicine as a diagnostic marker. Nomenclature The name γ-glutamyltransferase is preferred by the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology. The Expert Panel on Enzymes of the In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaline Phosphatase
The enzyme alkaline phosphatase (EC 3.1.3.1, alkaline phosphomonoesterase; phosphomonoesterase; glycerophosphatase; alkaline phosphohydrolase; alkaline phenyl phosphatase; orthophosphoric-monoester phosphohydrolase (alkaline optimum), systematic name phosphate-monoester phosphohydrolase (alkaline optimum)) catalyses the following reaction: : a phosphate monoester + H2O = an alcohol + phosphate Alkaline phosphatase has the physiological role of dephosphorylating compounds. The enzyme is found across a multitude of organisms, prokaryotes and eukaryotes alike, with the same general function but in different structural forms suitable to the environment they function in. Alkaline phosphatase is found in the periplasmic space of '' E. coli'' bacteria. This enzyme is heat stable and has its maximum activity at high pH. In humans, it is found in many forms depending on its origin within the body – it plays an integral role in metabolism within the liver and development withi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biomolecule
A biomolecule or biological molecule is a loosely used term for molecules present in organisms that are essential to one or more typically biological processes, such as cell division, morphogenesis, or development. Biomolecules include large macromolecules (or polyelectrolytes) such as proteins, carbohydrates, lipids, and nucleic acids, as well as small molecules such as primary metabolites, secondary metabolites and natural products. A more general name for this class of material is biological materials. Biomolecules are an important element of living organisms, those biomolecules are often endogenous, produced within the organism but organisms usually need exogenous biomolecules, for example certain nutrients, to survive. Biology and its subfields of biochemistry and molecular biology study biomolecules and their reactions. Most biomolecules are organic compounds, and just four elements—oxygen, carbon, hydrogen, and nitrogen—make up 96% of the human body's mass. But ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eukaryote
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bacteria and Archaea (both prokaryotes) make up the other two domains. The eukaryotes are usually now regarded as having emerged in the Archaea or as a sister of the Asgard archaea. This implies that there are only two domains of life, Bacteria and Archaea, with eukaryotes incorporated among archaea. Eukaryotes represent a small minority of the number of organisms, but, due to their generally much larger size, their collective global biomass is estimated to be about equal to that of prokaryotes. Eukaryotes emerged approximately 2.3–1.8 billion years ago, during the Proterozoic eon, likely as flagellated phagotrophs. Their name comes from the Greek εὖ (''eu'', "well" or "good") and κάρυον (''karyon'', "nut" or "kernel"). Euka ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prokaryote
A prokaryote () is a single-celled organism that lacks a nucleus and other membrane-bound organelles. The word ''prokaryote'' comes from the Greek πρό (, 'before') and κάρυον (, 'nut' or 'kernel').Campbell, N. "Biology:Concepts & Connections". Pearson Education. San Francisco: 2003. In the two-empire system arising from the work of Édouard Chatton, prokaryotes were classified within the empire Prokaryota. But in the three-domain system, based upon molecular analysis, prokaryotes are divided into two domains: ''Bacteria'' (formerly Eubacteria) and ''Archaea'' (formerly Archaebacteria). Organisms with nuclei are placed in a third domain, Eukaryota. In the study of the origins of life, prokaryotes are thought to have arisen before eukaryotes. Besides the absence of a nucleus, prokaryotes also lack mitochondria, or most of the other membrane-bound organelles that characterize the eukaryotic cell. It was once thought that prokaryotic cellular components within the cytop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Racemization
In chemistry, racemization is a conversion, by heat or by chemical reaction, of an optically active compound into a racemic (optically inactive) form. This creates a 1:1 molar ratio of enantiomers and is referred too as a racemic mixture (i.e. contain equal amount of (+) and (−) forms). Plus and minus forms are called Dextrorotation and levorotation. The D and L enantiomers are present in equal quantities, the resulting sample is described as a racemic mixture or a racemate. Racemization can proceed through a number of different mechanisms, and it has particular significance in pharmacology as different enantiomers may have different pharmaceutical effects. Stereochemistry Chiral molecules have two forms (at each point of asymmetry), which differ in their optical characteristics: The ''levorotatory form'' (the ''(−)-form'') will rotate counter-clockwise on the plane of polarization of a beam of light, whereas the ''dextrorotatory'' form (the ''(+)-form'') will rotate clockw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Racemic Mixture
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. History The first known racemic mixture was racemic acid, which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid. He manually separated the crystals of a mixture by hand, starting from an aqueous solution of the sodium ammonium salt of racemate tartaric acid. Pasteur benefited from the fact that ammonium tartrate salt that gives enantiomeric crystals with distinct crystal forms (at 77 °F). Reasoning from the macroscopic scale down to the molecular, he reckoned that the molecules had to have non-superimposable mirror images. A sample with only a single enantiomer is an ''enantiomerically pure'' or ''enantiopure'' compound. Etymology From racemic acid found in grapes; from Latin ''racemus'', meani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |