|
Nuclear Reactor Coolant
A nuclear reactor coolant is a coolant in a nuclear reactor used to remove heat from the nuclear reactor core and transfer it to electrical generators and the Environment (systems), environment. Frequently, a chain of two coolant loops are used because the primary coolant loop takes on short-term radioactivity from the reactor. Water Almost all currently operating nuclear power plants are light water reactors using ordinary water under high pressure as coolant and neutron moderator. About 1/3 are boiling water reactors where the primary coolant undergoes phase transition to steam inside the reactor. About 2/3 are pressurized water reactors at even higher pressure. Current reactors stay under the critical point (thermodynamics), critical point at around 374 °C and 218 Bar (unit), bar where the distinction between liquid and gas disappears, which limits thermal efficiency, but the proposed supercritical water reactor would operate above this point. Heavy water reactors use de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melting Point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa. When considered as the temperature of the reverse change from liquid to solid, it is referred to as the freezing point or crystallization point. Because of the ability of substances to supercool, the freezing point can easily appear to be below its actual value. When the "characteristic freezing point" of a substance is determined, in fact, the actual methodology is almost always "the principle of observing the disappearance rather than the formation of ice, that is, the melting point." Examples For most substances, melting and freezing points are approximately equal. For example, the melting point ''and'' freezing point of mercury is . How ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
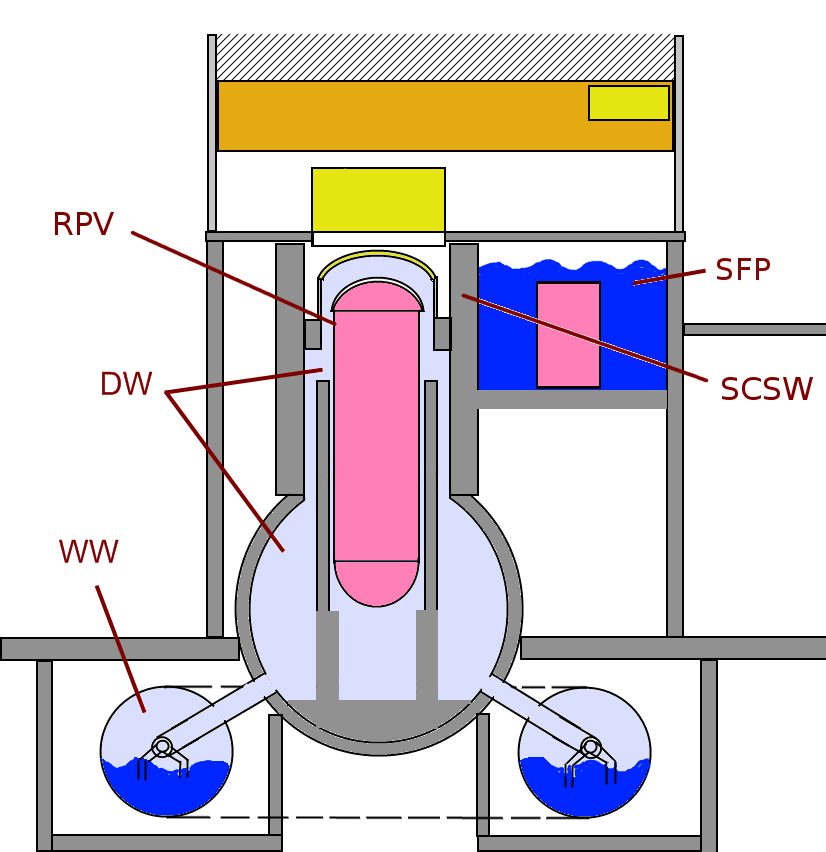
Boiling Water Reactor
A boiling water reactor (BWR) is a type of light water nuclear reactor used for the generation of electrical power. It is a design different from a Soviet graphite-moderated RBMK. It is the second most common type of electricity-generating nuclear reactor after the pressurized water reactor (PWR), which is also a type of light water nuclear reactor. The main difference between a BWR and PWR is that in a BWR, the reactor core heats water, which turns to steam and then drives a steam turbine. In a PWR, the reactor core heats water, which does not boil. This hot water then exchanges heat with a lower pressure system, which turns water into steam that drives the turbine. The BWR was developed by the Argonne National Laboratory and General Electric (GE) in the mid-1950s. The main present manufacturer is GE Hitachi Nuclear Energy, which specializes in the design and construction of this type of reactor. Overview A boiling water reactor uses demineralized water as a coolant and neu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fukushima Daiichi Nuclear Disaster
The was a nuclear accident in 2011 at the Fukushima Daiichi Nuclear Power Plant in Ōkuma, Fukushima, Japan. The proximate cause of the disaster was the 2011 Tōhoku earthquake and tsunami, which occurred on the afternoon of 11 March 2011 and remains the most powerful earthquake ever recorded in Japan. The earthquake triggered a powerful tsunami, with 13–14-meter-high waves damaging the nuclear power plant's emergency diesel generators, leading to a loss of electric power. The result was the most severe nuclear accident since the Chernobyl disaster in 1986, classified as level seven on the International Nuclear Event Scale (INES) after initially being classified as level five, and thus joining Chernobyl as the only other accident to receive such classification. While the 1957 explosion at the Mayak facility was the second worst by radioactivity released, the INES ranks incidents by impact on population, so Chernobyl (335,000 people evacuated) and Fukushima (154,000 evacuate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tritium
Tritium ( or , ) or hydrogen-3 (symbol T or H) is a rare and radioactive isotope of hydrogen with half-life about 12 years. The nucleus of tritium (t, sometimes called a ''triton'') contains one proton and two neutrons, whereas the nucleus of the common isotope hydrogen-1 (''protium'') contains one proton and zero neutrons, and that of hydrogen-2 (''deuterium'') contains one proton and one neutron. Naturally occurring tritium is extremely rare on Earth. The atmosphere has only trace amounts, formed by the interaction of its gases with cosmic rays. It can be produced artificially by irradiating lithium metal or lithium-bearing ceramic pebbles in a nuclear reactor and is a low-abundance byproduct in normal operations of nuclear reactors. Tritium is used as the energy source in radioluminescent lights for watches, gun sights, numerous instruments and tools, and even novelty items such as self-illuminating key chains. It is used in a medical and scientific setting as a radioacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tritium
Tritium ( or , ) or hydrogen-3 (symbol T or H) is a rare and radioactive isotope of hydrogen with half-life about 12 years. The nucleus of tritium (t, sometimes called a ''triton'') contains one proton and two neutrons, whereas the nucleus of the common isotope hydrogen-1 (''protium'') contains one proton and zero neutrons, and that of hydrogen-2 (''deuterium'') contains one proton and one neutron. Naturally occurring tritium is extremely rare on Earth. The atmosphere has only trace amounts, formed by the interaction of its gases with cosmic rays. It can be produced artificially by irradiating lithium metal or lithium-bearing ceramic pebbles in a nuclear reactor and is a low-abundance byproduct in normal operations of nuclear reactors. Tritium is used as the energy source in radioluminescent lights for watches, gun sights, numerous instruments and tools, and even novelty items such as self-illuminating key chains. It is used in a medical and scientific setting as a radioacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, which are repelled electrostatically. Neutron capture plays a significant role in the cosmic nucleosynthesis of heavy elements. In stars it can proceed in two ways: as a rapid process (r-process) or a slow process (s-process). Nuclei of masses greater than 56 cannot be formed by thermonuclear reactions (i.e., by nuclear fusion) but can be formed by neutron capture. Neutron capture on protons yields a line at 2.223 MeV predicted and commonly observed in solar flares. Neutron capture at small neutron flux At small neutron flux, as in a nuclear reactor, a single neutron is captured by a nucleus. For example, when natural gold (197Au) is irradiated by neutrons (n), the isotope 198Au is formed in a highly excited state, and quickly deca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deuterium
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two Stable isotope ratio, stable isotopes of hydrogen (the other being Hydrogen atom, protium, or hydrogen-1). The atomic nucleus, nucleus of a deuterium atom, called a deuteron, contains one proton and one neutron, whereas the far more common protium has no neutrons in the nucleus. Deuterium has a natural abundance in Earth's oceans of about one atom of deuterium among all atoms of hydrogen (see heavy water). Thus deuterium accounts for approximately 0.0156% by number (0.0312% by mass) of all the naturally occurring hydrogen in the oceans, while protium accounts for more than 99.98%. The abundance of deuterium changes slightly from one kind of natural water to another (see Vienna Standard Mean Ocean Water). (Tritium is yet another hydrogen isotope, with two neutrons, that is far more rare and is radioactive.) The name ''deuterium'' is derived from the Greek , meaning "second", to denot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heavy Water Reactor
A pressurized heavy-water reactor (PHWR) is a nuclear reactor that uses heavy water ( deuterium oxide D2O) as its coolant and neutron moderator. PHWRs frequently use natural uranium as fuel, but sometimes also use very low enriched uranium. The heavy water coolant is kept under pressure to avoid boiling, allowing it to reach higher temperature (mostly) without forming steam bubbles, exactly as for pressurized water reactor. While heavy water is very expensive to isolate from ordinary water (often referred to as ''light water'' in contrast to ''heavy water''), its low absorption of neutrons greatly increases the neutron economy of the reactor, avoiding the need for enriched fuel. The high cost of the heavy water is offset by the lowered cost of using natural uranium and/or alternative fuel cycles. As of the beginning of 2001, 31 PHWRs were in operation, having a total capacity of 16.5 GW(e), representing roughly 7.76% by number and 4.7% by generating capacity of all current o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supercritical Water Reactor
The supercritical water reactor (SCWR) is a concept Generation IV reactor, designed as a light water reactor (LWR) that operates at supercritical pressure (i.e. greater than 22.1 MPa). The term ''critical'' in this context refers to the critical point of water, and must not be confused with the concept of criticality of the nuclear reactor. The water heated in the reactor core becomes a supercritical fluid above the critical temperature of 374 °C, transitioning from a fluid more resembling liquid water to a fluid more resembling saturated steam (which can be used in a steam turbine), without going through the distinct phase transition of boiling. In contrast, the well-established pressurized water reactors (PWR) have a primary cooling loop of liquid water at a subcritical pressure, transporting heat from the reactor core to a secondary cooling loop, where the steam for driving the turbines is produced in a boiler (called the steam generator). Boiling water reactor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Efficiency
In thermodynamics, the thermal efficiency (\eta_) is a dimensionless performance measure of a device that uses thermal energy, such as an internal combustion engine, steam turbine, steam engine, boiler, furnace, refrigerator, ACs etc. For a heat engine, thermal efficiency is the ratio of the net work output to the heat input; in the case of a heat pump, thermal efficiency (known as the ''coefficient of performance'') is the ratio of net heat output (for heating), or the net heat removed (for cooling) to the energy input (external work). The efficiency of a heat engine is fractional as the output is always less than the input while the COP of a heat pump is more than 1. These values are further restricted by the Carnot theorem. Overview In general, energy conversion efficiency is the ratio between the useful output of a device and the input, in energy terms. For thermal efficiency, the input, Q_, to the device is heat, or the heat-content of a fuel that is consumed. The des ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bar (unit)
The bar is a metric unit of pressure, but not part of the International System of Units (SI). It is defined as exactly equal to 100,000 Pa (100 kPa), or slightly less than the current average atmospheric pressure on Earth at sea level (approximately 1.013 bar). By the barometric formula, 1 bar is roughly the atmospheric pressure on Earth at an altitude of 111 metres at 15 °C. The bar and the millibar were introduced by the Norwegian meteorologist Vilhelm Bjerknes, who was a founder of the modern practice of weather forecasting. The International System of Units, despite previously mentioning the bar, now omits any mention of it.. The bar has been legally recognised in countries of the European Union since 2004.British Standard BS 350:2004 ''Conversion Factors for Units''. The US National Institute of Standards and Technology (NIST) deprecates its use except for "limited use in meteorology" and lists it as one of several units that "must not be introduced ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Critical Point (thermodynamics)
In thermodynamics, a critical point (or critical state) is the end point of a phase equilibrium curve. The most prominent example is the liquid–vapor critical point, the end point of the pressure–temperature curve that designates conditions under which a liquid and its vapor can coexist. At higher temperatures, the gas cannot be liquefied by pressure alone. At the critical point, defined by a ''critical temperature'' ''T''c and a ''critical pressure'' ''p''c, phase boundaries vanish. Other examples include the liquid–liquid critical points in mixtures, and the ferromagnet–paramagnet transition (Curie temperature) in the absence of an external magnetic field. Liquid–vapor critical point Overview For simplicity and clarity, the generic notion of ''critical point'' is best introduced by discussing a specific example, the vapor–liquid critical point. This was the first critical point to be discovered, and it is still the best known and most studied one. The figu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |