|
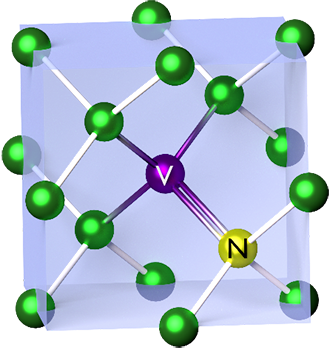
Nitrogen-vacancy Center
The nitrogen-vacancy center (N-V center or NV center) is one of numerous photoluminescent point defects in diamond. Its most explored and useful properties include its spin-dependent photoluminescence (which enables measurement of the electronic spin state using optically detected magnetic resonance), and its relatively long (millisecond) spin coherence at room temperature. The NV center energy levels are modified by magnetic fields, electric fields, temperature, and strain, which allow it to serve as a sensor of a variety of physical phenomena. Its atomic size and spin properties can form the basis for useful quantum sensors. It has also been explored for applications in quantum computing (e.g. for entanglement generation), quantum simulation, and spintronics. Structure The nitrogen-vacancy center is a point defect in the diamond lattice. It consists of a nearest-neighbor pair of a nitrogen atom, which substitutes for a carbon atom, and a lattice vacancy. Two charge states ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen-vacancy Center
The nitrogen-vacancy center (N-V center or NV center) is one of numerous point defects in diamond. Its most explored and useful property is its photoluminescence, which allows observers to read out its spin-state. The NV center's electron spin, localized at atomic scales, can be manipulated at room temperature by external factors such as magnetic, or electric fields, microwave radiation, or light, resulting in sharp resonances in the intensity of the photoluminescence. These resonances can be explained in terms of electron spin related phenomena such as quantum entanglement, spin–orbit interaction and Rabi oscillations, and analysed using advanced quantum optics theory. An individual NV center can be used as a basic unit for a quantum computer, a qubit, and used for quantum cryptography. Further potential applications in novel fields of electronics and sensing include spintronics, masers, and quantum sensors. If the charge is not specified the term "NV center" refers to the negativ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Optical Absorption
In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy — and so transforms electromagnetic energy into internal energy of the absorber (for example, thermal energy). A notable effect is attenuation, or the gradual reduction of the intensity of light waves as they propagate through a medium. Although the absorption of waves does not usually depend on their intensity (linear absorption), in certain conditions (optics) the medium's transparency changes by a factor that varies as a function of wave intensity, and saturable absorption (or nonlinear absorption) occurs. Quantifying absorption Many approaches can potentially quantify radiation absorption, with key examples following. * The absorption coefficient along with some closely related derived quantities * The attenuation coefficient (NB used infrequently with meaning synonymous with "absorption coefficient") * The Molar attenuation coefficient (als ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schottky Diode
The Schottky diode (named after the German physicist Walter H. Schottky), also known as Schottky barrier diode or hot-carrier diode, is a semiconductor diode formed by the junction of a semiconductor with a metal. It has a low forward voltage drop and a very fast switching action. The cat's-whisker detectors used in the early days of wireless and metal rectifiers used in early power applications can be considered primitive Schottky diodes. When sufficient forward voltage is applied, a current flows in the forward direction. A silicon p–n diode has a typical forward voltage of 600–700 mV, while the Schottky's forward voltage is 150–450 mV. This lower forward voltage requirement allows higher switching speeds and better system efficiency. History Walter H. Schottky (1886–1976) in 1914, discovered an irregularity in the emission of thermions in a vacuum tube, now known as the Schottky effect. Construction A metal–semiconductor junction is formed between a metal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermi Level
The Fermi level of a solid-state body is the thermodynamic work required to add one electron to the body. It is a thermodynamic quantity usually denoted by ''µ'' or ''E''F for brevity. The Fermi level does not include the work required to remove the electron from wherever it came from. A precise understanding of the Fermi level—how it relates to electronic band structure in determining electronic properties, how it relates to the voltage and flow of charge in an electronic circuit—is essential to an understanding of solid-state physics. In band structure theory, used in solid state physics to analyze the energy levels in a solid, the Fermi level can be considered to be a hypothetical energy level of an electron, such that at thermodynamic equilibrium this energy level would have a ''50% probability of being occupied at any given time''. The position of the Fermi level in relation to the band energy levels is a crucial factor in determining electrical properties. The Fermi le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physical Review B
''Physical Review B: Condensed Matter and Materials Physics'' (also known as PRB) is a peer-reviewed, scientific journal, published by the American Physical Society (APS). The Editor of PRB is Laurens W. Molenkamp. It is part of the ''Physical Review'' family of journals. About the Physical Review Journals The current Editor in Chief is . PRB currently publishes over 4500 papers a year, making it one of the largest physics journals in the world. PRB ranked by the Eigenfactor, University of Washingto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Symmetry
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of these molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can be used to predict or explain many of a molecule's chemical properties, such as whether or not it has a dipole moment, as well as its allowed spectroscopic transitions. To do this it is necessary to use group theory. This involves classifying the states of the molecule using the irreducible representations from the character table of the symmetry group of the molecule. Symmetry is useful in the study of molecular orbitals, with applications to the Hückel method, to ligand field theory, and to the Woodward-Hoffmann rules. Many university level textbooks on physical chemistry, quantum chemistry, spectroscopy and inorganic chemistry discuss symmetry. Another framework on a larger scale is the use of crystal systems to describe crystallographic symmetry in bulk materia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lone Pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone pairs are found in the outermost electron shell of atoms. They can be identified by using a Lewis structure. Electron pairs are therefore considered lone pairs if two electrons are paired but are not used in chemical bonding. Thus, the number of electrons in lone pairs plus the number of electrons in bonds equals the number of valence electrons around an atom. Lone pair is a concept used in valence shell electron pair repulsion theory (VSEPR theory) which explains the shapes of molecules. They are also referred to in the chemistry of Lewis acids and bases. However, not all non-bonding pairs of electrons are considered by chemists to be lone pairs. Examples are the transition metals where the non-bonding pairs do not influence molecular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Covalent Bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding. Covalent bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, bent bonds, three-center two-electron bonds and three-center four-electron bonds. The term ''covalent bond'' dates from 1939. The prefix ''co-'' means ''jointly, associated in action, partnered to a lesser degree, '' etc.; thus a "co-valent bond", in essence, means that the atoms share " valence", such a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physical Review Letters
''Physical Review Letters'' (''PRL''), established in 1958, is a peer-reviewed, scientific journal that is published 52 times per year by the American Physical Society. As also confirmed by various measurement standards, which include the ''Journal Citation Reports'' impact factor and the journal ''h''-index proposed by Google Scholar, many physicists and other scientists consider ''Physical Review Letters'' to be one of the most prestigious journals in the field of physics. ''According to Google Scholar, PRL is the journal with the 9th journal h-index among all scientific journals'' ''PRL'' is published as a print journal, and is in electronic format, online and CD-ROM. Its focus is rapid dissemination of significant, or notable, results of fundamental research on all topics related to all fields of physics. This is accomplished by rapid publication of short reports, called "Letters". Papers are published and available electronically one article at a time. When published in s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reports On Progress In Physics
''Reports on Progress in Physics'' is a monthly peer review, peer-reviewed scientific journal published by IOP Publishing. The editor-in-chief as of 2022 is Subir Sachdev (Harvard University). Scope The focus of this journal is invited review articles covering all branches of physics. Each review will typically survey and critique a particular topic, or developments in a field. Introductions of articles are intended for a broad readership, beyond the specialist or expert. In addition to the traditional review article two other formats are available: ''Reports on Progress'' (about 20 pages) and ''Key Issues Reviews'' (about 10 pages).Scope IOP. Retrieved on Sep. 5, 2016 Abstracting and indexing ''Reports on Progress in Physics'' is abstracted and indexed in the following databases:[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Paramagnetic Resonance
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spins excited are those of the electrons instead of the atomic nuclei. EPR spectroscopy is particularly useful for studying metal complexes and organic radicals. EPR was first observed in Kazan State University by Soviet physicist Yevgeny Zavoisky in 1944, and was developed independently at the same time by Brebis Bleaney at the University of Oxford. Theory Origin of an EPR signal Every electron has a magnetic moment and spin quantum number s = \tfrac , with magnetic components m_\mathrm = + \tfrac or m_\mathrm = - \tfrac . In the presence of an external magnetic field with strength B_\mathrm , the electron's magnetic moment aligns itself either antiparallel ( m_\mathrm = - \tfrac ) or parallel ( m_\mathrm = + \tfrac ) to the fie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |