|
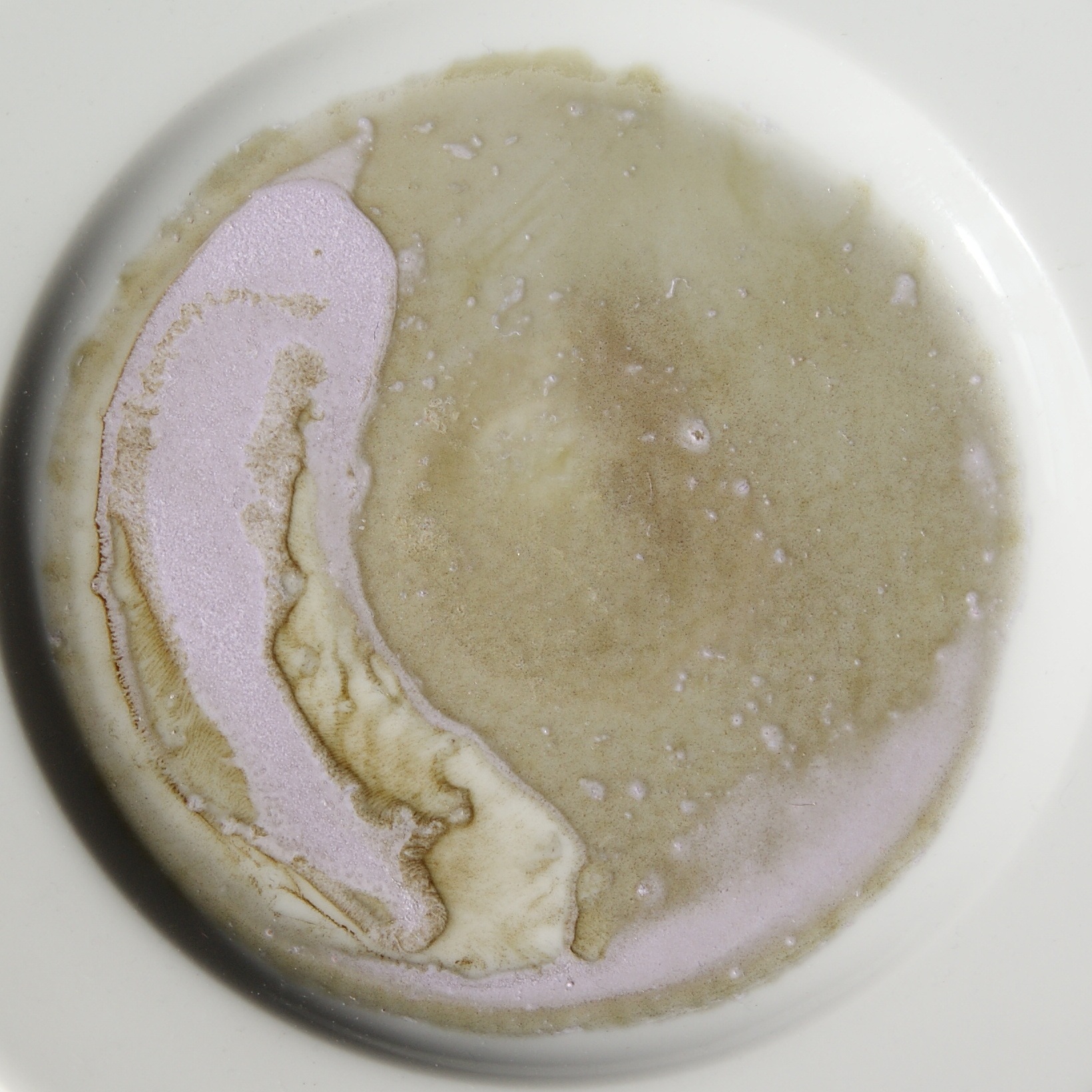
Neodymium Compounds
Neodymium compounds are compounds formed by the lanthanide metal neodymium (Nd). In these compounds, neodymium generally exhibits the +3 oxidation state, such as NdCl3, Nd2(SO4)3 and Nd(CH3COO)3. Compounds with neodymium in the +2 oxidation state are also known, such as NdCl2 and NdI2. Some neodymium compounds have colors that vary based upon the type of lighting.Burke M.W. (1996) Lighting II: Sources. In: Image Acquisition. Springer, Dordrecht. File:Neodymium tl1.jpg, Neodymium compounds in fluorescent tube light—from left to right, the sulfate, nitrate, and chloride File:Neodymium fluorescent1.jpg, Neodymium compounds in compact fluorescent lamp light File:Neodymium daylight1.jpg, Neodymium compounds in normal daylight Halides Neodymium can form four trihalides of the form NdX3. It reacts vigorously with all the stable halogens: : violet substance: mauve substance: violet substance: green substance The dihalides NdCl2 and NdBr2 are dark green solids,Georg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttrium, are often collectively known as the rare-earth elements or rare-earth metals. The informal chemical symbol Ln is used in general discussions of lanthanide chemistry to refer to any lanthanide. All but one of the lanthanides are f-block elements, corresponding to the filling of the 4f electron shell. There is some dispute on whether lanthanum or lutetium is a d-block element, but lutetium is usually considered so by those who study the matter; it is included due to its chemical similarities with the other 14. All lanthanide elements form trivalent cations, Ln3+, whose chemistry is largely determined by the ionic radius, which decreases steadily from lanthanum to lutetium. These elements are called lanthanides because the elements i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neodymium(II) Iodide
Neodymium(II) iodide or neodymium diiodide is an inorganic salt of iodine and neodymium the formula NdI2. Neodymium uses the +2 oxidation state in the compound. Neodymium(II) iodide is a violet solid. The compound is not stoichiometry, stoichiometric. It melting, melts at 562°C. Preparation Neodymium(II) iodide can be made by heating molten neodymium(III) iodide with neodymium metal at 800 and 580°C for 12 hours. It can also be obtained by reducing neodymium(III) iodide with neodymium in a vacuum at 800 to 900°C: :Nd + 2NdI3 → 3NdI2 The reaction of neodymium with mercury(II) iodide is also possible because neodymium is more reactive than mercury: :Nd + HgI2 → NdI2 + Hg Direct preparation from iodine and neodymium is also possible: :Nd + I2 → NdI2 The compound was first synthesized by John D. Corbett in 1961.Angelika Jungmann, R. Claessen, R. Zimmermann, G. e. Meng, P. Steiner, S. Hüfner, S. Tratzky, K. Stöwe, H. P. Beck: ''Photoemission of LaI2 and CeI2.'' In: ''Z ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neodymium(III) Acetate
Neodymium acetate is an inorganic salt composed of a neodymium atom trication and three acetate groups as anions where neodymium exhibits the +3 oxidation state. It has a chemical formula of Nd(CH3COO)3 although it can be informally referred to as NdAc because Ac is an informal symbol for acetate. It commonly occurs as a light purple powder.National Center for Biotechnology Information (2022). PubChem Compound Summary for CID 3563803, Neodymium acetate. Retrieved April 10, 2022 from https://pubchem.ncbi.nlm.nih.gov/compound/Neodymium-acetate Physical properties Neodymium acetate as a hydrate is a purple solid that is soluble in water. The solubility of the compound increases when sodium acetate is added, forming a blue complex. It forms crystalline hydrates in the composition of Nd(CH3COO)3·''n''H2O, where n = 1 and 4 are red-violet crystals that lose water at 110 °C. The crystalline hydrate with the composition of Nd(CH3COO)3·4H2O forms crystals of triclinic crystal sys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Bicarbonate
Ammonium bicarbonate is an inorganic compound with formula (NH4)HCO3. The compound has many names, reflecting its long history. Chemically speaking, it is the bicarbonate salt of the ammonium ion. It is a colourless solid that degrades readily to carbon dioxide, water and ammonia. Production Ammonium bicarbonate is produced by combining carbon dioxide and ammonia: :CO2 + NH3 + H2O → (NH4)HCO3 Since ammonium bicarbonate is thermally unstable, the reaction solution is kept cold, which allows the precipitation of the product as white solid. About 100,000 tons were produced in this way in 1997. Ammonia gas passed into a strong aqueous solution of the sesquicarbonate (a 2:1:1 mixture of (NH4)HCO3, (NH4)2CO3, and H2O) converts it into normal ammonium carbonate ((NH4)2CO3), which can be obtained in the crystalline condition from a solution prepared at about 30 °C. This compound on exposure to air gives off ammonia and reverts to ammonium bicarbonate. Salt of hartshorn Compositions co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neodymium(III) Carbonate
Neodymium(III) carbonate is an inorganic compound, a salt, where neodymium is in the +3 oxidation state and the carbonate ion is in the -2 oxidation state.See https://pubchem.ncbi.nlm.nih.gov/compound/Neodymium_III_-carbonate-hydrate It has a chemical formula of Nd2(CO3)3. The anhydrous form is purple-red,Rare earth elements: Main volume, Phần 3 (Leopold Gmelin; Verlag Chemie, 1994), page 22; 68. Retrieved 4 February 2021. while the octahydrate is a pink solid.Handbook… (Pierre Villars, Karin Cenzual, Roman Gladyshevskii; Walter de Gruyter GmbH & Co KG, 24 thg 7, 2017 - 1970 pages) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neodymium(III) Oxalate
Neodymium(III) oxalate is the oxalate salt of neodymium, with the chemical formula of Nd2(C2O4)3 in the anhydrous or hydrate form. Its decahydrate decomposes to the anhydrous form when heated, and when heated further, decomposes to Nd2O2C2O4, finally obtaining neodymium(III) oxide. It dissolves in hydrochloric acid Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid Acid strength is the tendency of an acid, symbol ... to form Nd(C2O4)Cl·3H2O.Moebius, R.; Matthes, F. The exchange of oxalate ions for chloride ions of the oxalate hydrates of the rare earths and yttrium. ''Zeitschrift für Chemie'', 1964. 4 (6): 234-235. ISSN: 0044-2402. References {{Oxalates Neodymium compounds Oxalates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neodymium(III) Arsenate
Neodymium arsenate, also known as neodymium(III) arsenate, is the arsenate of neodymium with the chemical formula of NdAsO4. In this compound, neodymium exhibits the +3 oxidation state. It has good thermal stability, and its p''K''sp,c is 21.86±0.11. Preparation Neodymium arsenate can be obtained from the reaction between sodium arsenate (Na3AsO4) and neodymium chloride (NdCl3) in solution:Gabisoniya, Ts. D.; Nanobashvili, E. M.. Synthesis of rare earth metal arsenates. ''Soobshcheniya Akademii Nauk Gruzinskoi SSR'' (1980), 97(2), 345-8. : Na3AsO4 + NdCl3 → 3 NaCl + NdAsO4↓ See also * Arsenic Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, but ... References Neodymium compounds Arsenates {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neodymium(III) Chloride
Neodymium(III) chloride or neodymium trichloride is a chemical compound of neodymium and chlorine with the formula NdCl3. This anhydrous compound is a mauve-colored solid that rapidly absorbs water on exposure to air to form a purple-colored hexa hydrate, NdCl3·6H2O. Neodymium(III) chloride is produced from minerals monazite and bastnäsite using a complex multistage extraction process. The chloride has several important applications as an intermediate chemical for production of neodymium metal and neodymium-based lasers and optical fibers. Other applications include a catalyst in organic synthesis and in decomposition of waste water contamination, corrosion protection of aluminium and its alloys, and fluorescent labeling of organic molecules ( DNA). Appearance NdCl3 is a mauve colored hygroscopic solid whose color changes to purple upon absorption of atmospheric water. The resulting hydrate, like many other neodymium salts, has the interesting property that it appears diff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% , it is referred to as ''fuming nitric acid''. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%. Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as pigments in inks and dyes. Nitric acid is also commonly used as a strong oxidizing agen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neodymium(III) Nitrate
Neodymium nitrate is a chemical compound with the formula Nd(NO3)3. It is typically encountered as the hexahydrate, Nd(NO3)3·6H2O, which is more accurately formulated as d(NO3)3(H2O)42H2O to reflect the crystal structure. It decomposes to NdONO3 at elevated temperature. This water-soluble salt finds use in fabrication of perovskite (CaTiO3) based solid oxide fuel cells, synthesis of Nd3+ doped vanadium pentoxide (V2O5) nanostructure for potential usage in supercapacitors and as a catalyst for Friedlander synthesis of surface modified quinolones Quinolone may refer to: * 2-Quinolone * 4-Quinolone 4-Quinolone is an organic compound derived from quinoline. It and 2-quinolone are the two most important parent (meaning simplified) quinolones. 4-Quinolone exists in equilibrium with a mino ... for application in medicinal chemistry. References External links Neodymium Nitrate at americanelements.com Neodymium compounds Nitrates {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formula . It is a colorless, odorless and viscous liquid that is miscible with water. Pure sulfuric acid does not exist naturally on Earth due to its strong affinity to water vapor; it is hygroscopic and readily absorbs water vapor from the air. Concentrated sulfuric acid is highly corrosive towards other materials, from rocks to metals, since it is an oxidant with powerful dehydrating properties. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid, but to the contrary dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus the reverse procedure of adding water to the acid should not be performed since the heat released may boi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |