|
Nelson Complexity Index
The Nelson complexity index (NCI) is a measure to compare the secondary conversion capacity of a petroleum refinery with the primary distillation capacity. The index provides an easy metric for quantifying and ranking the complexity of various refineries and units. To calculate the index, it is necessary to use complexity factors, which compare the cost of upgrading units to the cost of crude distillation unit. History It was developed by Wilbur L. Nelson in a series of articles that appeared in the '' Oil & Gas Journal'' from 1960 to 1961 (Mar. 14, p. 189; Sept. 26, p. 216; and June 19, p. 109). In 1976, he elaborated on the concept in another series of articles, again in the ''Oil & Gas Journal'' (Sept. 13, p. 81; Sept. 20, p. 202; and Sept. 27, p. 83). Formula \text = \sum_^N F_i \cdot \frac Where: *F_i is a complexity factor *C_i is a unit capacity *C_\text is a capacity of crude distillation unit *N is a number of all units The NCI assigns ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vacuum Distillation
Vacuum distillation is distillation performed under reduced pressure, which allows the purification of compounds not readily distilled at ambient pressures or simply to save time or energy. This technique separates compounds based on differences in their boiling points. This technique is used when the boiling point of the desired compound is difficult to achieve or will cause the compound to decompose. Reduced pressures decrease the boiling point of compounds. The reduction in boiling point can be calculated using a temperature-pressure nomograph using the Clausius–Clapeyron relation. Laboratory-scale applications Compounds with a boiling point lower than 150 °C typically are distilled at ambient pressure. For samples with high boiling points, short-path distillation apparatus is commonly employed. This technique is amply illustrated in Organic Synthesis. Rotary evaporation Rotary evaporation is a common technique used in laboratories to concentrate or isolate a comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomerisation
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomerization. When the isomerization occurs intramolecularly it may be called a rearrangement reaction. When the activation energy for the isomerization reaction is sufficiently small, both isomers will exist in a temperature-dependent equilibrium with each other. Many values of the standard free energy difference, \Delta G^\circ, have been calculated, with good agreement between observed and calculated data. Examples and applications Alkanes Skeletal isomerization occurs in the cracking process, used in the petrochemical industry. As well as reducing the average chain length, straight-chain hydrocarbons are converted to branched isomers in the process, as illustrated the following reaction of ''n''-butane to ''i''-butane. :\overset -> \ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatics
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past grouping of molecules based on smell, before their general chemical properties are understood. The current definition of aromatic compounds does not have any relation with their smell. Heteroarenes are closely related, since at least one carbon atom of CH group is replaced by one of the heteroatoms oxygen, nitrogen, or sulfur. Examples of non-benzene compounds with aromatic properties are furan, a heterocyclic compound with a five-membered ring that includes a single oxygen atom, and pyridine, a heterocyclic compound with a six-membered ring containing one nitrogen atom. Hydrocarbons without an aromatic ring are called aliphatic. Benzene ring model Benzene, C6H6, is the least complex aromatic hydrocarbon, and it was the first one named as suc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents. Nucleophilic alkylating agents Nucleophilic alkylating agents deliver the equivalent of an alkyl anion ( carbanion). The formal "alkyl anion" attacks an electrophile, forming a new coval ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalytic Hydrocracking
In petrochemistry, petroleum geology and organic chemistry, cracking is the process whereby complex organic molecules such as kerogens or long-chain hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of carbon-carbon bonds in the precursors. The rate of cracking and the end products are strongly dependent on the temperature and presence of catalysts. Cracking is the breakdown of a large alkane into smaller, more useful alkenes. Simply put, hydrocarbon cracking is the process of breaking a long chain of hydrocarbons into short ones. This process requires high temperatures. More loosely, outside the field of petroleum chemistry, the term "cracking" is used to describe any type of splitting of molecules under the influence of heat, catalysts and solvents, such as in processes of destructive distillation or pyrolysis. Fluid catalytic cracking produces a high yield of petrol and LPG, while hydrocracking is a major source of jet fuel, d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalytic Cracking
Fluid Catalytic Cracking (FCC) is the conversion process used in petroleum refineries to convert the high-boiling point, high-molecular weight hydrocarbon fractions of petroleum (crude oils) into gasoline, olefinic gases, and other petroleum products. The cracking of petroleum hydrocarbons was originally done by thermal cracking, now virtually replaced by catalytic cracking, which yields greater volumes of high octane rating gasoline; and produces by-product gases, with more carbon-carbon double bonds (i.e. olefins), that are of greater economic value than the gases produced by thermal cracking. The feedstock to the FCC conversion process usually is heavy gas oil (HGO), which is that portion of the petroleum (crude oil) that has an initial boiling-point temperature of or higher, at atmospheric pressure, and that has an average molecular weight that ranges from about 200 to 600 or higher; heavy gas oil also is known as “heavy vacuum gas oil” (HVGO). In the fluid catalytic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalytic Reforming
Catalytic reforming is a chemical process used to convert petroleum refinery naphthas distilled from crude oil (typically having low octane ratings) into high-octane liquid products called reformates, which are premium blending stocks for high-octane gasoline. The process converts low-octane linear hydrocarbons (paraffins) into branched alkanes (isoparaffins) and cyclic naphthenes, which are then partially dehydrogenated to produce high-octane aromatic hydrocarbons. The dehydrogenation also produces significant amounts of byproduct hydrogen gas, which is fed into other refinery processes such as hydrocracking. A side reaction is hydrogenolysis, which produces light hydrocarbons of lower value, such as methane, ethane, propane and butanes. In addition to a gasoline blending stock, reformate is the main source of aromatic bulk chemicals such as benzene, toluene, xylene and ethylbenzene which have diverse uses, most importantly as raw materials for conversion into plastics. Howev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Asphalt
Asphalt, also known as bitumen (, ), is a sticky, black, highly viscous liquid or semi-solid form of petroleum. It may be found in natural deposits or may be a refined product, and is classed as a pitch. Before the 20th century, the term asphaltum was also used. Full text at Internet Archive (archive.org) The word is derived from the Ancient Greek ἄσφαλτος ''ásphaltos''. The largest natural deposit of asphalt in the world, estimated to contain 10 million tons, is the Pitch Lake located in La Brea in southwest Trinidad ( Antilles island located on the northeastern coast of Venezuela), within the Siparia Regional Corporation. The primary use (70%) of asphalt is in road construction, where it is used as the glue or binder mixed with aggregate particles to create asphalt concrete. Its other main uses are for bituminous waterproofing products, including production of roofing felt and for sealing flat roofs. In material sciences and engineering, the terms "asphal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
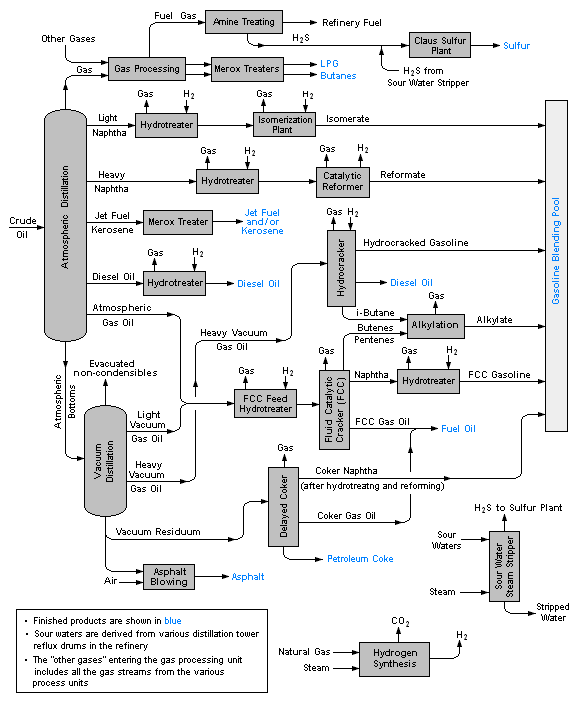
Petroleum Refinery
An oil refinery or petroleum refinery is an industrial process plant where petroleum (crude oil) is transformed and refined into useful products such as gasoline (petrol), diesel fuel, asphalt base, fuel oils, heating oil, kerosene, liquefied petroleum gas and petroleum naphtha. Petrochemicals feedstock like ethylene and propylene can also be produced directly by cracking crude oil without the need of using refined products of crude oil such as naphtha. The crude oil feedstock has typically been processed by an oil production plant. There is usually an oil depot at or near an oil refinery for the storage of incoming crude oil feedstock as well as bulk liquid products. In 2020, the total capacity of global refineries for crude oil was about 101.2 million barrels per day. Oil refineries are typically large, sprawling industrial complexes with extensive piping running throughout, carrying streams of fluids between large chemical processing units, such as distillation columns. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reliance Industries Limited
Reliance Industries Limited is an Indian multinational conglomerate company, headquartered in Mumbai. It has diverse businesses including energy, petrochemicals, natural gas, retail, telecommunications, mass media, and textiles. Reliance is one of the most profitable companies in India, the largest publicly traded company in India by market capitalisation, and the largest company in India as measured by revenue. It is also the one of the top largest employer in India with over 236,000 employees in the world. The company is ranked 100th on the Fortune Global 500 list of the world's biggest corporations as of 2022. Reliance continues to be India's largest exporter, accounting for 7% of India's total merchandise exports and it has access to markets in over 100 countries. Reliance is responsible for almost 5% of the Government of India's total revenue from customs and excise duty. It is also the highest income tax payer in the private sector in India. The company has relatively ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jamnagar Refinery
The Jamnagar Refinery is the private sector crude oil refinery owned by Reliance Industries in Jamnagar, Gujarat, India. The refinery was commissioned on 14 July 1999 with an installed capacity of . Its current installed capacity is . It is currently the largest refinery in the world. History On 25 December 2008, Reliance Petroleum Limited (RPL) announced the commissioning of its refinery into a Special Economic Zone in Jamnagar district of Gujarat, India. The completion of the RPL (Reliance Petroleum limited) refinery has enabled Jamnagar to emerge as a 'Refinery land', housing the world's largest refining complex with an aggregate refining capacity of of oil per day, more than any other single location in the world. The globally competitive RPL refinery was commissioned in 36 months. RPL contracted several companies having expertise in engineering construction and refining like Bechtel, UOP LLC and Foster Wheeler (Multinational Company)amongst others. There were plans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |