|
Nanoparticle Deposition
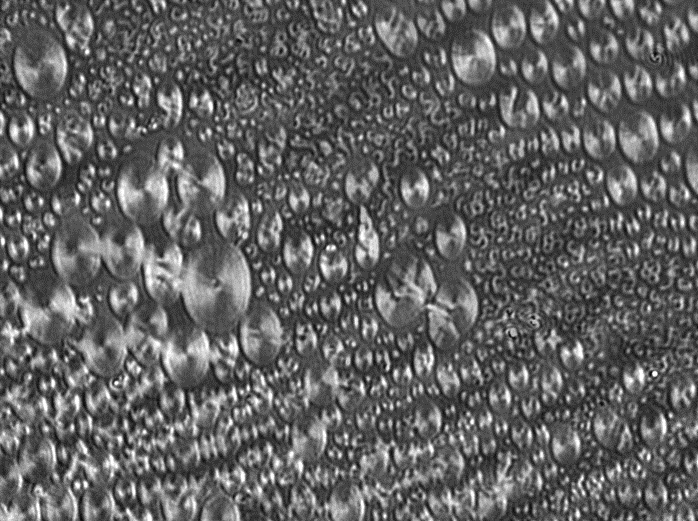
Nanoparticle deposition refers to the process of attaching nanoparticles to solid surfaces called substrates to create coatings of nanoparticles. The coatings can have a monolayer or a multilayer and organized or unorganized structure based on the coating method used. Nanoparticles are typically difficult to deposit due to their physical properties. Challenges Nanoparticles can be made from different materials such as metals, ceramics and polymers. The stability of the nanoparticles can be an issue as nanoparticles have a tendency to lower their very high surface energy, which originates from their high surface-to-bulk ratio. Bare nanoparticles tend to stabilize themselves either by sorption of molecules from the surroundings or by lowering the surface area through coagulation and agglomeration. Usually the formation of these aggregates is unwanted. The tendency of a nanoparticle to coagulate can be controlled by modifying the surface layer. In a liquid medium, suitable ligand mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nanoparticle Coating
A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two directions. At the lowest range, metal particles smaller than 1 nm are usually called atom clusters instead. Nanoparticles are usually distinguished from microparticles (1-1000 µm), "fine particles" (sized between 100 and 2500 nm), and "coarse particles" (ranging from 2500 to 10,000 nm), because their smaller size drives very different physical or chemical properties, like colloidal properties and ultrafast optical effects or electric properties. Being more subject to the brownian motion, they usually do not sediment, like colloidal particles that conversely are usually understood to range from 1 to 1000 nm. Being much smaller than the wavelengths of visible light (400-700 nm), nanopa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nanoparticle
A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two directions. At the lowest range, metal particles smaller than 1 nm are usually called atom clusters instead. Nanoparticles are usually distinguished from microparticles (1-1000 µm), "fine particles" (sized between 100 and 2500 nm), and "coarse particles" (ranging from 2500 to 10,000 nm), because their smaller size drives very different physical or chemical properties, like colloidal properties and ultrafast optical effects or electric properties. Being more subject to the brownian motion, they usually do not sediment, like colloidal particles that conversely are usually understood to range from 1 to 1000 nm. Being much smaller than the wavelengths of visible light (400-700 nm), nano ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coating
A coating is a covering that is applied to the surface of an object, usually referred to as the Substrate (materials science), substrate. The purpose of applying the coating may be decorative, functional, or both. Coatings may be applied as liquids, gases or solids e.g. Powder coatings. Paints and lacquers are coatings that mostly have dual uses of protecting the substrate and being decorative, although some artists paints are only for decoration, and the paint on large industrial pipes is for preventing corrosion and identification e.g. blue for process water, red for fire-fighting control etc. Functional coatings may be applied to change the surface properties of the substrate, such as adhesion, Wetting, wettability, corrosion resistance, or wear resistance. In other cases, e.g. semiconductor device fabrication (where the substrate is a wafer (electronics), wafer), the coating adds a completely new property, such as a magnetic response or electrical conductivity, and forms an e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monolayer
A monolayer is a single, closely packed layer of atoms, molecules, or cells. In some cases it is referred to as a self-assembled monolayer. Monolayers of layered crystals like graphene and molybdenum disulfide are generally called 2D materials. Chemistry A Langmuir monolayer or insoluble monolayer is a one-molecule thick layer of an insoluble organic material spread onto an aqueous subphase in a Langmuir-Blodgett trough. Traditional compounds used to prepare Langmuir monolayers are amphiphilic materials that possess a hydrophilic headgroup and a hydrophobic tail. Since the 1980s a large number of other materials have been employed to produce Langmuir monolayers, some of which are semi-amphiphilic, including polymeric, ceramic or metallic nanoparticles and macromolecules such as polymers. Langmuir monolayers are extensively studied for the fabrication of Langmuir-Blodgett film (LB films), which are formed by transferred monolayers on a solid substrate. A Gibbs monolayer or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Energy
In surface science, surface free energy (also interfacial free energy or surface energy) quantifies the disruption of intermolecular bonds that occurs when a surface is created. In solid-state physics, surfaces must be intrinsically less energetically favorable than the bulk of the material (the atoms on the surface have more energy compared with the atoms in the bulk), otherwise there would be a driving force for surfaces to be created, removing the bulk of the material (see sublimation). The surface energy may therefore be defined as the excess energy at the surface of a material compared to the bulk, or it is the work required to build an area of a particular surface. Another way to view the surface energy is to relate it to the work required to cut a bulk sample, creating two surfaces. There is "excess energy" as a result of the now-incomplete, unrealized bonding at the two surfaces. Cutting a solid body into pieces disrupts its bonds and increases the surface area, and th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sorption
Sorption is a physical and chemical process by which one substance becomes attached to another. Specific cases of sorption are treated in the following articles: ; Absorption: "the incorporation of a substance in one state into another of a different state" (e.g., liquids being absorbed by a solid or gases being absorbed by a liquid); ; Adsorption: The physical adherence or bonding of ions and molecules onto the surface of another phase (e.g., reagents adsorbed to a solid catalyst surface); ; Ion exchange: An exchange of ions between two electrolytes or between an electrolyte solution and a complex. The reverse of sorption is desorption. Sorption rate The adsorption and absorption rate of a diluted solute in gas or liquid solution to a surface or interface can be calculated using Fick's laws of diffusion. See also * Sorption isotherm Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Langmuir–Blodgett Film
A Langmuir–Blodgett (LB) film is a nanostructured system formed when Langmuir films—or Langmuir monolayers (LM)—are transferred from the liquid-gas interface to solid supports during the vertical passage of the support through the monolayers. LB films can contain one or more monolayers of an organic material, deposited from the surface of a liquid onto a solid by immersing (or emersing) the solid substrate into (or from) the liquid. A monolayer is adsorbed homogeneously with each immersion or emersion step, thus films with very accurate thickness can be formed. This thickness is accurate because the thickness of each monolayer is known and can therefore be added to find the total thickness of a Langmuir–Blodgett film. The monolayers are assembled vertically and are usually composed either of amphiphilic molecules (see chemical polarity) with a hydrophilic head and a hydrophobic tail (example: fatty acids) or nowadays commonly of nanoparticles. Langmuir–Blodgett films ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
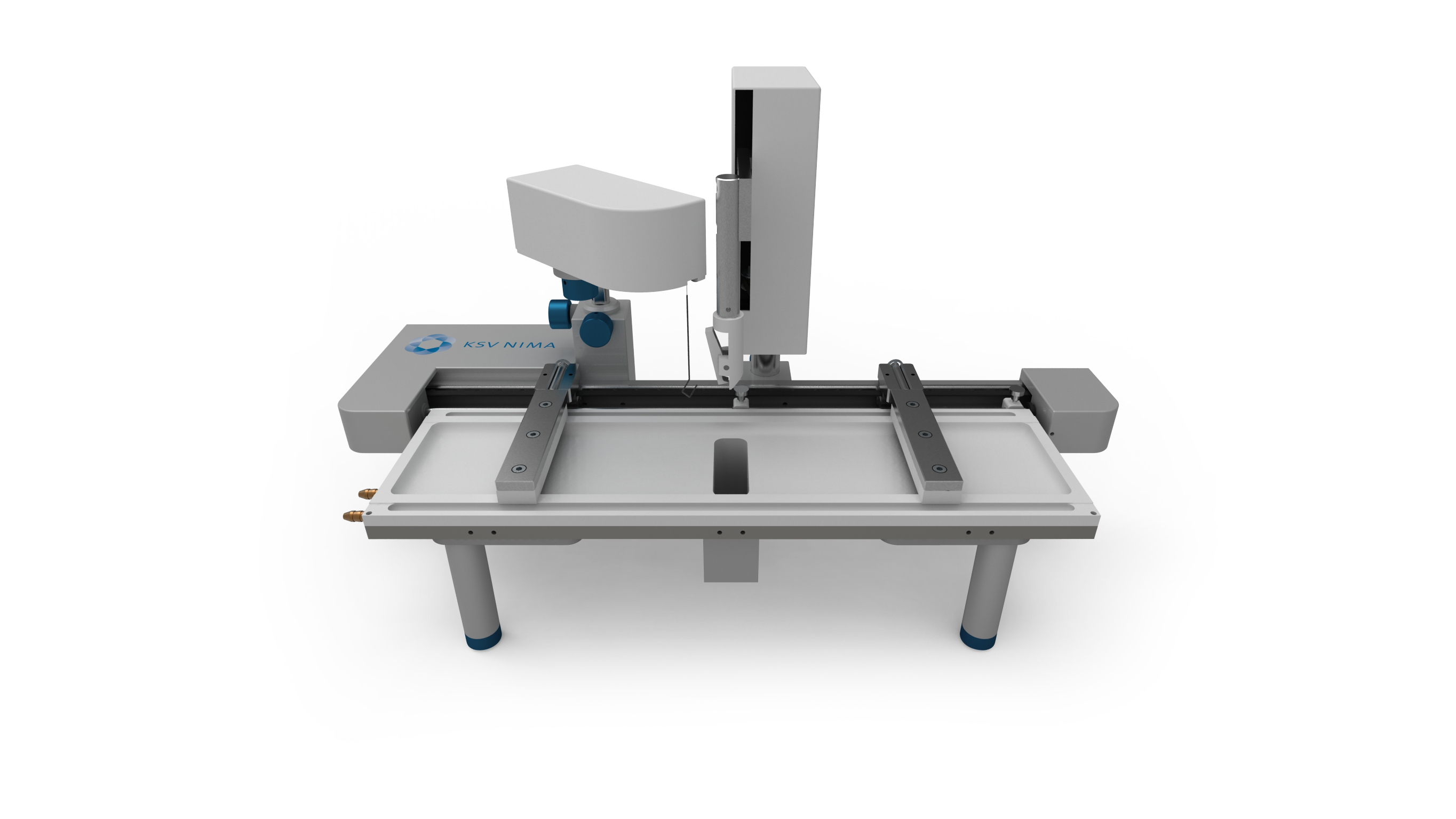
Langmuir–Blodgett Trough
A Langmuir–Blodgett trough (LB trough) is a laboratory apparatus that is used to compress monolayers of molecules on the surface of a given subphase (usually water) and measures surface phenomena due to this compression. It can also be used to deposit single or multiple monolayers on a solid substrate. Description Overview The idea of a Langmuir–Blodgett (LB) film was first proven feasible in 1917 when Irving Langmuir (Langmuir, 1917) showed that single water-surface monolayers could be transferred to solid substrates. 18 years later, Katharine Blodgett made an important scientific advance when she discovered that several of these single monolayer films could be stacked on top of one another to make multilayer films (Blodgett 1935). Since then, LB films (and subsequently the troughs to make them) have been used for a wide variety of scientific experimentation, ranging from 2D crystallization of proteins to Brewster angle microscopy. The LB trough's general objective is to st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brewster Angle Microscope
A Brewster angle microscope (BAM) is a microscope for studying thin films on liquid surfaces, most typically Langmuir films. In a Brewster angle microscope, both the microscope and a polarized light source are aimed towards a liquid surface at that liquid's Brewster angle, in such a way for the microscope to catch an image of any light reflected from the light source via the liquid surface. Because there is no ''p''-polarized reflection from the pure liquid when both are angled towards it at the Brewster angle, light is only reflected when some other phenomenon such as a surface film affects the liquid surface. The technique was first introduced in 1991. Applications Brewster angle microscopes enable the visualization of Langmuir monolayers or adsorbate films at the air-water interface for example as a function of packing density. They can be used either to study the properties of the Langmuir layer, or to indicate a suitable deposition pressure for Langmuir-Blodgett (LB) de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
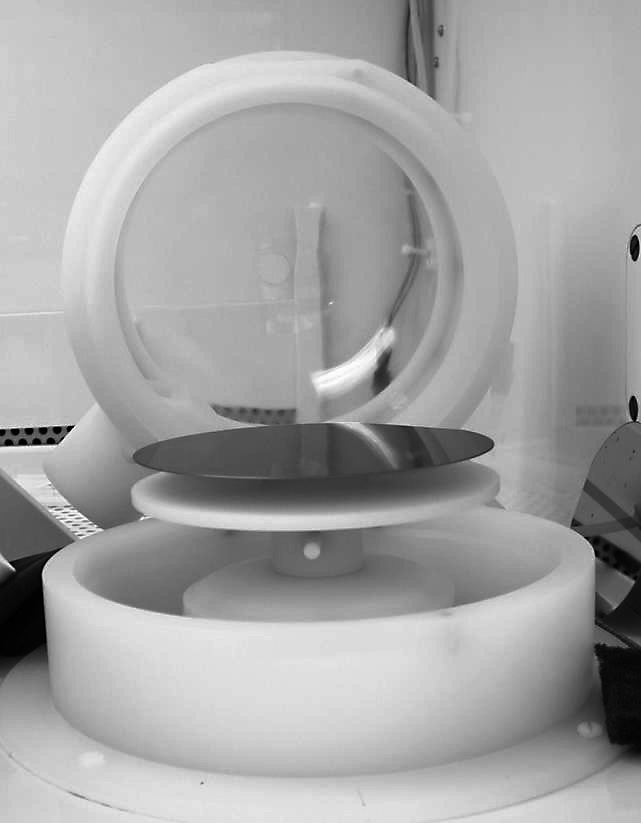
Spin Coating
Spin coating is a procedure used to deposit uniform thin films onto flat substrates. Usually a small amount of coating material is applied on the center of the substrate, which is either spinning at low speed or not spinning at all. The substrate is then rotated at speed up to 10,000 rpm to spread the coating material by centrifugal force. A machine used for spin coating is called a spin coater, or simply spinner. Rotation is continued while the fluid spins off the edges of the substrate, until the desired thickness of the film is achieved. The applied solvent is usually volatile, and simultaneously evaporates. The higher the angular speed of spinning, the thinner the film. The thickness of the film also depends on the viscosity and concentration of the solution, and the solvent. Pioneering theoretical analysis of spin coating was undertaken by Emslie et al., and has been extended by many subsequent authors (including Wilson et al., who studied the rate of spreading in spin coati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dip-coating
image:Dip coating.svg, A schematic of the continuous dip coating process. Roll of coarse cloth Cloth Bath Liquid material Rollers Oven Scrapers Excess liquid falls back A coating remains on the fabric cloth. Dip coating is an industrial coating process which is used, for example, to manufacture bulk products such as coated fabrics and condoms and specialised coatings for example in the biomedical field. Dip coating is also commonly used in academic research, where many chemical and nano material engineering research projects use the dip coating technique to create thin-film coatings. The earliest dip-coated products may have been candles. For flexible laminar substrates such as fabrics, dip coating may be performed as a continuous roll-to-roll process. For coating a 3D object, it may simply be inserted and removed from the bath of coating. For condom-making, a former is dipped into the coating. For some products, such as early methods of making candles, the process is re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Vapor Deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films. In typical CVD, the wafer (substrate) is exposed to one or more volatile precursors, which react and/or decompose on the substrate surface to produce the desired deposit. Frequently, volatile by-products are also produced, which are removed by gas flow through the reaction chamber. Microfabrication processes widely use CVD to deposit materials in various forms, including: monocrystalline, polycrystalline, amorphous, and epitaxial. These materials include: silicon ( dioxide, carbide, nitride, oxynitride), carbon (fiber, nanofibers, nanotubes, diamond and graphene), fluorocarbons, filaments, tungsten, titanium nitride and various high-κ dielectrics. The term ''chemical vapour deposition'' was coined 1960 by ''John M. Blocher, Jr.'' who intended to differentiate ''chemic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |