|
Nitrilotriacetic Acid
Nitrilotriacetic acid (NTA) is the aminopolycarboxylic acid with the formula N(CH2CO2H)3. It is a colourless solid that is used as a chelating agent, which forms coordination compounds with metal ions (chelates) such as Ca2+, Co2+, Cu2+, and Fe3+. Production and use Nitrilotriacetic acid is commercially available as the free acid and as the sodium salt. It is produced from ammonia, formaldehyde, and sodium cyanide or hydrogen cyanide. Worldwide capacity is estimated at 100 thousand tonnes per year. NTA is also cogenerated as an impurity in the synthesis of EDTA, arising from reactions of the ammonia coproduct.Hart, J. Roger (2005) "Ethylenediaminetetraacetic Acid and Related Chelating Agents" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim. Older routes to NTA included alkylation of ammonia with chloroacetic acid and oxidation of triethanolamine. Coordination chemistry and applications NTA is a tripodal tetradentate trianionic ligand. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society (professional association) in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 34,000 in the UK and a further 8,000 abroad. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
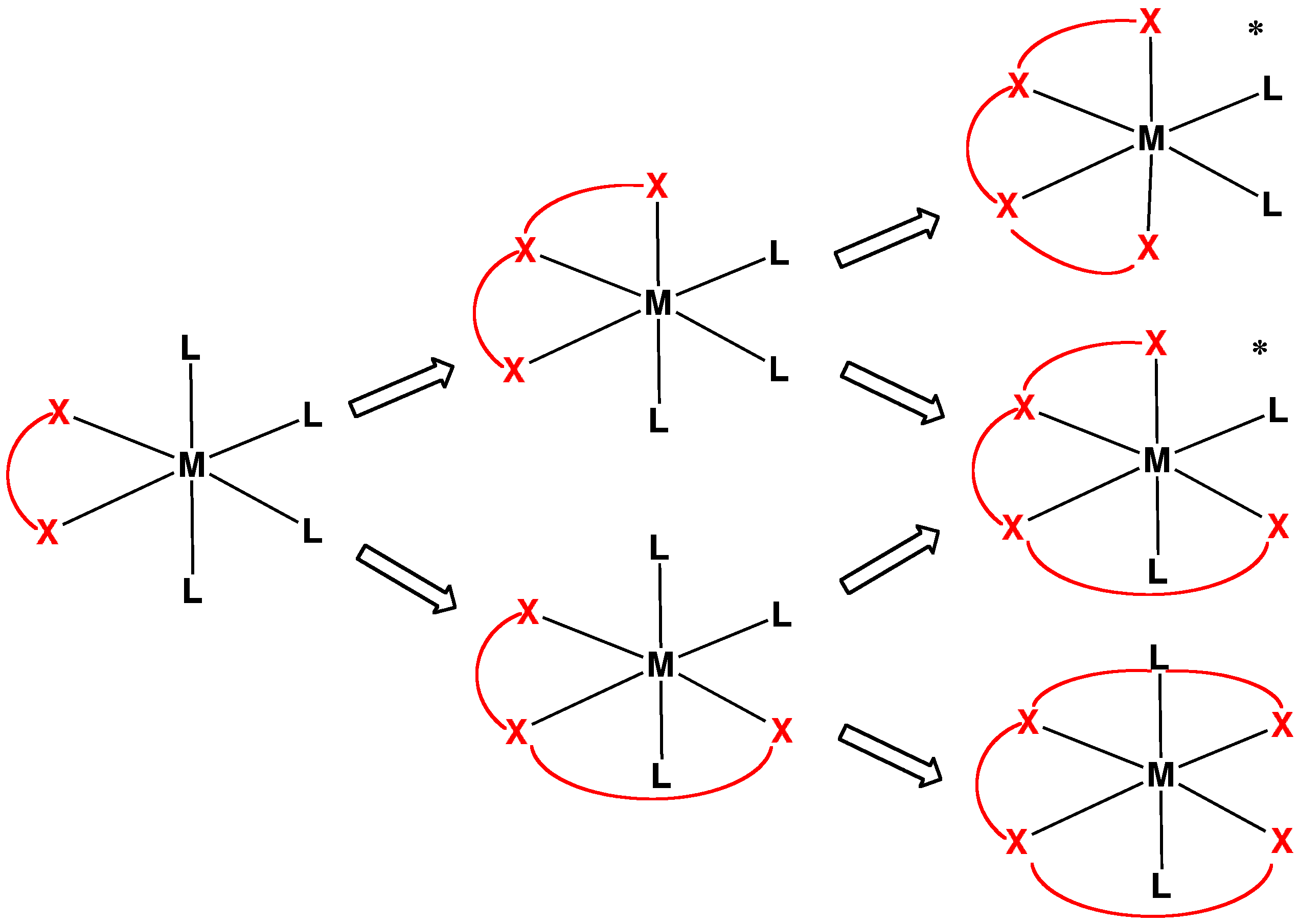
Tetradentate Ligand
In chemistry, tetradentate ligands are ligands that bind four donor atoms to a central atom to form a coordination complex. This number of donor atoms that bind is called denticity and is a method of classifying ligands. Tetradentate ligands are common in nature in the form of chlorophyll, which has a core ligand called chlorin, and heme, which has a core ligand called porphyrin. They are responsible for the colour observed in plants and humans. Phthalocyanine is an artificial macrocyclic tetradentate ligand that is used to make blue and green pigments. Shape Tetradentate ligands can be classified by the topology of the connections between donor atoms. Common forms are linear (also called sequential), ring or tripodal. A tetrapodal ligand that is also tetradentate has four legs with donor atoms and a bridgehead that is not a donor. Upon binding with a central atom, there are several arrangements possible (known as geometric isomers). Linear ligands A linear tetradentate ligand ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chelating Agents
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity, as in the case of zinc and its use as a maintenance therapy to prevent the absorption of copper in people with Wilson's disease. Chelation is useful in applications such as providing nutritional supplements, in chelation therapy to remove toxic metals from the body, as contrast agents in MRI scanning, in manufacturing using homogeneous catalysts, in chemical water treatment to assist in the removal of metals, and in fertilizers. Chelate effect The chelate effect is the greater affinity of chelating ligands for a metal ion than that of similar nonchelating (monodentate) ligands for the same metal. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Acids
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements. Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important chemical reagent and industrial chemical, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is fundamental to all forms of life. When bound to coenzyme A, it is central to the metabolism of carbohydrates and fats. The global demand for acetic acid is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amines
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. Aroma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agarose
Agarose is a heteropolysaccharide, generally extracted from certain red seaweed. It is a linear polymer made up of the repeating unit of agarobiose, which is a disaccharide made up of D-galactose and 3,6-anhydro-L-galactopyranose. Agarose is one of the two principal components of agar, and is purified from agar by removing agar's other component, agaropectin. Agarose is frequently used in molecular biology for the separation of large molecules, especially DNA, by electrophoresis. Slabs of agarose gels (usually 0.7 - 2%) for electrophoresis are readily prepared by pouring the warm, liquid solution into a mold. A wide range of different agaroses of varying molecular weights and properties are commercially available for this purpose. Agarose may also be formed into beads and used in a number of chromatographic methods for protein purification. Structure Agarose is a linear polymer with a molecular weight of about 120,000, consisting of alternating D- galactose and 3,6-an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iminodiacetic Acid
Iminodiacetic acid is the organic compound with the formula HN(CH2CO2H)2, often abbreviated to IDA. A white solid, the compound is a dicarboxylic acid amine (the nitrogen atom forms a secondary amino group, not an imino group as the name suggests). The iminodiacetate dianion is a tridentate ligand, forming metal complexes by forming two, fused, five membered chelate rings. The proton on the nitrogen atom can be replaced by a carbon atom of a polymer to create an ion-exchange resin, such as chelex 100. Complexes of IDA and EDTA were introduced in the early 1950's by Schwarzenbach. IDA forms stronger complexes than the bidentate ligand glycine and weaker complexes than the tetradentate ligand nitrilotriacetic acid. It can also act as a bidentate ligand through its two carboxylate groups. Several technetium-99''m'' complexes are used in cholescintigraphy scans (also known as ''hepatobiliary iminodiacetic acid scans'') to evaluate the health and function of the gallbladder. Im ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chelation
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity, as in the case of zinc and its use as a maintenance therapy to prevent the absorption of copper in people with Wilson's disease. Chelation is useful in applications such as providing nutritional supplements, in chelation therapy to remove toxic metals from the body, as contrast agents in MRI scanning, in manufacturing using homogeneous catalysts, in chemical water treatment to assist in the removal of metals, and in fertilizers. Chelate effect The chelate effect is the greater affinity of chelating ligands for a metal ion than that of similar nonchelating (monodentate) ligands for the same metal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to react with air under standard conditions because a passivation layer of nickel oxide forms on the surface that prevents further corrosion. Even so, pure native nickel is found in Earth's crust only in tiny amounts, usually in ultramafic rocks, and in the interiors of larger nickel–iron meteorites that were not exposed to oxygen when outside Earth's atmosphere. Meteoric nickel is found in combination with iron, a reflection of the origin of those elements as major end products of supernova nucleosynthesis. An iron–nickel mixture is thought to compose Earth's outer and inner cores. Use of nickel (as natural meteoric nickel–iron alloy) has been traced as far back as 3500 BCE. Nickel was first isolated and classified a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyhistidine-tag
A polyhistidine-tag is an amino acid motif in proteins that typically consists of at least six histidine (''His'') residues, often at the N- or C-terminus of the protein. It is also known as hexa histidine-tag, 6xHis-tag, His6 tag, by the US trademarked name HIS TAG (US Trademark serial number 74242707), and most commonly as His-Tag. The tag was invented by Roche, although the use of histidines and its vectors are distributed by Qiagen. Various purification kits for histidine-tagged proteins are available from Qiagen, Sigma, Thermo Scientific, GE Healthcare, Macherey-NagelCube Biotech Clontech, Bio-Radand others. MK(HQ)6 may be used for enhanced expression in ''E. coli'' and tag removal. The total number of histidine residues may vary in the tag from as low as two, to as high as 10 or more His residues. N- or C-terminal His-tags may also be followed or preceded, respectively, by a suitable amino acid sequence that facilitates removal of the polyhistidine-tag using endopepti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromated Copper Arsenate
Chromated copper arsenate (CCA) is a wood preservative containing compounds of chromium, copper, and arsenic, in various proportions. It is used to impregnate timber and other wood products, especially those intended for outdoor use, in order to protect them from attack by microbes and insects. Like other copper-based wood preservatives, it imparts a greenish tint to treated timber. CCA was invented in 1933 by Indian chemist Sonti Kamesam, and patented in Britain in 1934. It has been used for timber treatment since the mid-1930s, and is marketed under many trade names. In 2003, the United States Environmental Protection Agency and the lumber industry agreed to discontinue the use of CCA-treated wood in most residential construction. This agreement was intended to protect the health of humans and the environment by reducing exposure to the arsenic in CCA-treated wood. As a result of this decision, CCA-treated wood can no longer be used to construct residential structures such as pl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphosphate
Polyphosphates are salts or esters of polymeric oxyanions formed from tetrahedral PO4 ( phosphate) structural units linked together by sharing oxygen atoms. Polyphosphates can adopt linear or a cyclic ring structures. In biology, the polyphosphate esters ADP and ATP are involved in energy storage. A variety of polyphosphates find application in mineral sequestration in municipal waters, generally being present at 1 to 5 ppm. GTP, CTP, and UTP are also nucleotides important in the protein synthesis, lipid synthesis, and carbohydrate metabolism, respectively. Polyphosphates are also used as food additives, marked E452. Structure Image:Triphosphorsäure.svg, Structure of triphosphoric acid Image:Polyphosphoric acid.svg, Polyphosphoric acid Image:Trimetaphosphat.svg, Cyclic tri metaphosphate Image:Adenosindiphosphat protoniert.svg, Adenosine diphosphate (ADP) The structure of tripolyphosphoric acid illustrates the principles which define the structures of po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |