|
N-Propyl Iodide
''n''-Propyl iodide (also 1-propyl iodide or 1-iodopropane) is a colorless, flammable chemical compound. It has the chemical formula C3H7I and is prepared by heating ''n''-propyl alcohol with iodine and phosphorus.''Merck Index ''The Merck Index'' is an encyclopedia of chemicals, drugs and biologicals with over 10,000 monograph on single substances or groups of related compounds published online by the Royal Society of Chemistry. History The first edition of the Mer ...'', 9th ed., monograph 7651 References Iodoalkanes Propyl compounds {{Organohalide-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intraperitoneal
The peritoneum is the serous membrane forming the lining of the abdominal cavity or coelom in amniotes and some invertebrates, such as annelids. It covers most of the intra-abdominal (or coelomic) organs, and is composed of a layer of mesothelium supported by a thin layer of connective tissue. This peritoneal lining of the cavity supports many of the abdominal organs and serves as a conduit for their blood vessels, lymphatic vessels, and nerves. The abdominal cavity (the space bounded by the vertebrae, abdominal muscles, diaphragm, and pelvic floor) is different from the intraperitoneal space (located within the abdominal cavity but wrapped in peritoneum). The structures within the intraperitoneal space are called "intraperitoneal" (e.g., the stomach and intestines), the structures in the abdominal cavity that are located behind the intraperitoneal space are called "retroperitoneal" (e.g., the kidneys), and those structures below the intraperitoneal space are called "subperito ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intravenous
Intravenous therapy (abbreviated as IV therapy) is a medical technique that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutrients for those who cannot, or will not—due to reduced mental states or otherwise—consume food or water by mouth. It may also be used to administer medications or other medical therapy such as blood products or electrolytes to correct electrolyte imbalances. Attempts at providing intravenous therapy have been recorded as early as the 1400s, but the practice did not become widespread until the 1900s after the development of techniques for safe, effective use. The intravenous route is the fastest way to deliver medications and fluid replacement throughout the body as they are introduced directly into the circulatory system and thus quickly distributed. For this reason, the intravenous route of administration is also used for the consumpti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Iodide
Ethyl iodide (also iodoethane) is a colorless flammable chemical compound. It has the chemical formula C2H5I and is prepared by heating ethanol with iodine and phosphorus.''Merck Index of Chemicals and Drugs'', 9th ed., monograph 3753 On contact with air, especially on the effect of light, it decomposes and turns yellow or reddish from dissolved iodine. It may also be prepared by reaction between hydroiodic acid and ethanol distilling off the ethyl iodide. Ethyl iodide should be stored in the presence of copper powder to avoid rapid decomposition, though even with this method samples do not last more than 1 year. Because iodide is a good leaving group, ethyl iodide is an excellent ethylating agent. It is also used as the hydrogen radical promoter. Production Ethyl iodide is prepared by using red phosphorus, absolute ethanol and iodine. The iodine dissolves in the ethanol, where it reacts with the solid phosphorus to form phosphorus triiodide Phosphorus triiodide (PI3) is an i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopropyl Iodide
Isopropyl iodide is the organoiodine compound with the formula (CH3)2CHI. It is colorless, flammable, and volatile. Organic iodides are light-sensitive and take on a yellow colour upon storage, owing to the formation of iodine. Preparation Isopropyl iodide is prepared by iodination of isopropyl alcohol using hydrogen iodide or, equivalently, with a mixture of glycerol, iodine, and phosphorus.Merck Index of Chemicals and Drugs, 9th ed., monograph 5074 An alternative preparation involves the reaction of 2-propyl bromide with an acetone solution of sodium iodide (Finkelstein reaction The Finkelstein reaction named after the German chemist Hans Finkelstein, is an SN2 reaction (Substitution Nucleophilic Bimolecular reaction) that involves the exchange of one halogen atom for another. It is an equilibrium reaction, but the react ...):Textbook of Practical Organic Chemistry, 5th Edition, Prentice Hall, 1989 :(CH3)2CHBr + NaI → (CH3)2CHI + NaBr References {{Organohalide-st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butyl Iodide
Butyl iodide (1-iodobutane) is an organic compound which is an iodo derivative of butane. It is used as an alkylating agent Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting al .... Isomer The compound isobutyl iodide AKA 1-iodo-2-methylpropane is isomeric to butyl iodide. References Alkylating agents Iodoalkanes {{Organohalide-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propane
Propane () is a three-carbon alkane with the molecular formula . It is a gas at standard temperature and pressure, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as a fuel in domestic and industrial applications and in low-emissions public transportation. Discovered in 1857 by the French chemist Marcellin Berthelot, it became commercially available in the US by 1911. Propane is one of a group of liquefied petroleum gases (LP gases). The others include butane, propylene, butadiene, butylene, isobutylene, and mixtures thereof. Propane has lower volumetric energy density, but higher gravimetric energy density and burns more cleanly than gasoline and coal. Propane gas has become a popular choice for barbecues and portable stoves because its low −42 °C boiling point makes it vaporise inside pressurised liquid containers (2 phases). Propane powers buses, forklifts, taxis, outboard boat motors, and ic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-Propyl Chloride
''n''-Propyl chloride (also 1-propyl chloride or 1-chloropropane) is a colorless, flammable chemical compound. It has the chemical formula C3H7Cl and is prepared by reacting ''n''-propyl alcohol with phosphorus trichloride in the presence of a zinc chloride Zinc chloride is the name of inorganic chemical compounds with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. This salt is hygroscopic and ev ... catalyst.Merck Index of Chemicals and Drugs, 9th ed., monograph 7635 References Chloroalkanes {{organohalide-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-Propyl Bromide
1-Bromopropane (''n''-propylbromide or nPB) is an organobromine compound with the chemical formula CH3CH2CH2Br. It is a colorless liquid that is used as a solvent. It has a characteristic hydrocarbon odor. Its industrial applications increased dramatically in the 21st century due to the phasing out of chlorofluorocarbons, perchloroethylene, and chloroalkanes such as 1,1,1-Trichloroethane under the Montreal Protocol. Preparation Industrial routes to 1-bromopropane involve free-radical additions to the corresponding alkenes. In this way, the anti-Markovnikov product is obtained.David Ioffe, Arieh Kampf "Bromine, Organic Compounds" in Kirk-Othmer Encyclopedia of Chemical Technology 2002 by John Wiley & Sons. . A laboratory synthesis involves treating propanol with a mixture of hydrobromic and sulfuric acids: :CH3CH2CH2OH + HBr → CH3CH2CH2Br + H2O Alternate synthetic routes include treating propanol with phosphorus tribromide. or via a Hunsdiecker reaction with buty ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
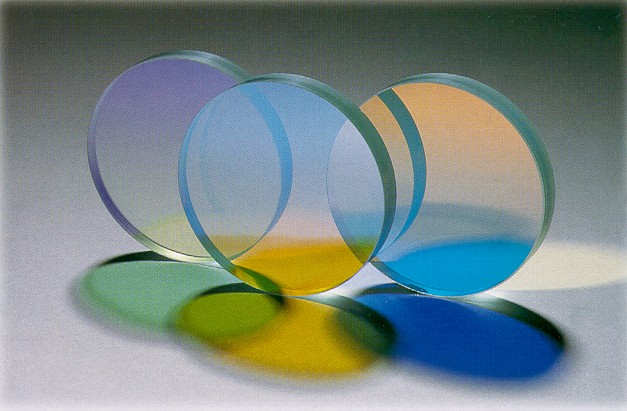
Transparency And Translucency
In the field of optics, transparency (also called pellucidity or diaphaneity) is the physical property of allowing light to pass through the material without appreciable scattering of light. On a macroscopic scale (one in which the dimensions are much larger than the wavelengths of the photons in question), the photons can be said to follow Snell's law. Translucency (also called translucence or translucidity) allows light to pass through, but does not necessarily (again, on the macroscopic scale) follow Snell's law; the photons can be scattered at either of the two interfaces, or internally, where there is a change in index of refraction. In other words, a translucent material is made up of components with different indices of refraction. A transparent material is made up of components with a uniform index of refraction. Transparent materials appear clear, with the overall appearance of one color, or any combination leading up to a brilliant spectrum of every color. The opposit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flammable
A combustible material is something that can burn (i.e., ''combust'') in air. A combustible material is flammable if it ignites easily at ambient temperatures. In other words, a combustible material ignites with some effort and a flammable material catches fire immediately on exposure to flame. The degree of flammability or combustibility in air depends largely upon the volatility of the material - this is related to its composition-specific vapour pressure, which is temperature dependent. The quantity of vapour produced can be enhanced by increasing the surface area of the material forming a mist or dust. Take wood as an example. Finely divided wood dust can undergo explosive combustion and produce a blast wave. A piece of paper (made from wood) catches on fire quite easily. A heavy oak desk is much harder to ignite, even though the wood fibre is the same in all three materials. Common sense (and indeed scientific consensus until the mid-1700s) would seem to suggest that ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propan-1-ol
Propan-1-ol (also propanol, n-propyl alcohol) is a primary alcohol with the formula and sometimes represented as PrOH or ''n''-PrOH. It is a colorless liquid and an isomer of 2-propanol. It is formed naturally in small amounts during many fermentation processes and used as a solvent in the pharmaceutical industry, mainly for resins and cellulose esters, and, sometimes, as a disinfecting agent. Chemical properties Propan-1-ol shows the normal reactions of a primary alcohol. Thus it can be converted to alkyl halides; for example red phosphorus and iodine produce n-propyl iodide in 80% yield, while with catalytic gives n-propyl chloride. Reaction with acetic acid in the presence of an catalyst under Fischer esterification conditions gives propyl acetate, while refluxing propanol overnight with formic acid alone can produce propyl formate in 65% yield. Oxidation of propan-1-ol with and gives a 36% yield of propionaldehyde, and therefore for this type of reaction higher yi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |