|
N-propyl Bromide
1-Bromopropane (''n''-propylbromide or nPB) is an organobromine compound with the chemical formula CH3CH2CH2Br. It is a colorless liquid that is used as a solvent. It has a characteristic hydrocarbon odor. Its industrial applications increased dramatically in the 21st century due to the phasing out of chlorofluorocarbons, perchloroethylene, and chloroalkanes such as 1,1,1-Trichloroethane under the Montreal Protocol. Preparation Industrial routes to 1-bromopropane involve free-radical additions to the corresponding alkenes. In this way, the anti-Markovnikov product is obtained.David Ioffe, Arieh Kampf "Bromine, Organic Compounds" in Kirk-Othmer Encyclopedia of Chemical Technology 2002 by John Wiley & Sons. . A laboratory synthesis involves treating propanol with a mixture of hydrobromic and sulfuric acids: :CH3CH2CH2OH + HBr → CH3CH2CH2Br + H2O Alternate synthetic routes include treating propanol with phosphorus tribromide. or via a Hunsdiecker reaction with but ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromoethane
Bromoethane, also known as ethyl bromide, is a chemical compound of the haloalkanes group. It is abbreviated by chemists as EtBr (which is also used as an abbreviation for ethidium bromide). This volatile compound has an ether-like odor. Preparation The preparation of EtBr stands as a model for the synthesis of bromoalkanes in general. It is usually prepared by the addition of hydrogen bromide to ethene: :H2C=CH2 + HBr → H3C-CH2Br Bromoethane is inexpensive and would rarely be prepared in the laboratory. A laboratory synthesis includes reacting ethanol with a mixture of hydrobromic and sulfuric acids. An alternate route involves refluxing ethanol with phosphorus and bromine; phosphorus tribromide is generated ''in situ''. Uses In organic synthesis, EtBr is the synthetic equivalent of the ethyl carbocation (Et+) synthon. In reality, such a cation is not actually formed. For example, carboxylates salts are converted to ethyl esters, carbanions to ethylated derivatives, thiou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butyric Acid
Butyric acid (; from grc, βούτῡρον, meaning "butter"), also known under the systematic name butanoic acid, is a straight-chain alkyl carboxylic acid with the chemical formula CH3CH2CH2CO2H. It is an oily, colorless liquid with an unpleasant odor. Isobutyric acid (2-methylpropanoic acid) is an isomer. Salts and esters of butyric acid are known as butyrates or butanoates. The acid does not occur widely in nature, but its esters are widespread. It is a common industrial chemical and an important component in the mammalian gut. History Butyric acid was first observed in impure form in 1814 by the French chemist Michel Eugène Chevreul. By 1818, he had purified it sufficiently to characterize it. However, Chevreul did not publish his early research on butyric acid; instead, he deposited his findings in manuscript form with the secretary of the Academy of Sciences in Paris, France. Henri Braconnot, a French chemist, was also researching the composition of butter and w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Personal Protective Equipment
Personal protective equipment (PPE) is protective clothing, helmets, goggles, or other garments or equipment designed to protect the wearer's body from injury or infection. The hazards addressed by protective equipment include physical, electrical, heat, chemicals, biohazards, and airborne particulate matter. Protective equipment may be worn for job-related occupational safety and health purposes, as well as for sports and other recreational activities. ''Protective clothing'' is applied to traditional categories of clothing, and ''protective gear'' applies to items such as pads, guards, shields, or masks, and others. PPE suits can be similar in appearance to a cleanroom suit. The purpose of personal protective equipment is to reduce employee exposure to hazards when engineering controls and administrative controls are not feasible or effective to reduce these risks to acceptable levels. PPE is needed when there are hazards present. PPE has the serious limitation t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
California Occupational Safety And Health Administration
The Division of Occupational Safety and Health of California (DOSH, but more commonly known as Cal/OSHA) is an agency of the Government of California established by the California Occupational Safety & Health Act of 1973. Administered by the California Department of Industrial Relations, Cal/OSHA's mission is to protect public health and safety through research and regulation related to hazards on the job in California workplaces as well as on elevators, amusement rides, and ski lifts, and related to the use of pressure vessels such as boilers and tanks. Cal/OSHA requires that qualifying organizations create illness and injury prevention programs meant to help identify and eliminate dangers before accidents and illnesses occur. As of December 22, 2015, Cal/OSHA employed 195 field enforcement officers, 25 of whom received bilingual pay for using a second language at least 10% of the time on the job. The organization offers training materials and paid training time to staff intereste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
American Conference Of Governmental Industrial Hygienists
The American Conference of Governmental Industrial Hygienists (ACGIH) is a professional association of industrial hygienists and practitioners of related professions, with headquarters in Cincinnati, Ohio. One of its goals is to advance worker protection by providing timely, objective, scientific information to occupational and environmental health professionals. History The National Conference of Governmental Industrial Hygienists (NCGIH) convened on June 27, 1938, in Washington, D.C. NCGIH's original constitution limited full membership to two representatives from each governmental industrial hygiene agency. Associate membership was made available to other professional personnel of the agencies holding full memberships, and also to personnel of educational institutions engaged in teaching industrial hygiene. Governmental industrial hygiene personnel of other countries were eligible for affiliated membership. The Conference came into being with 59 members, one affiliated me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toxic Substances Control Act Of 1976
The Toxic Substances Control Act (TSCA) is a United States law, passed by the 94th United States Congress in 1976 and administered by the United States Environmental Protection Agency (EPA), that regulates chemicals not regulated by other U.S. federal statutes, including chemicals already in commerce and the introduction of new chemicals.Auer, Charles, Frank Kover, James Aidala, Marks Greenwood“Toxic Substances: A Half Century of Progress.”EPA Alumni Association. March 2016. When the TSCA was put into place, all existing chemicals were considered to be safe for use and subsequently grandfathered in. Its three main objectives are to assess and regulate new commercial chemicals before they enter the market, to regulate chemicals already existing in 1976 that posed an "unreasonable risk of injury to health or the environment", as for example PCBs, lead, mercury and radon, and to regulate these chemicals' distribution and use. Contrary to what the name implies, TSCA does not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
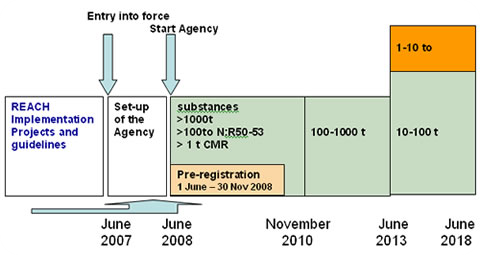
Registration, Evaluation, Authorisation And Restriction Of Chemicals
Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) is a European Union regulation dating from 18 December 2006. REACH addresses the production and use of chemical substances, and their potential impacts on both human health and the environment. Its 849 pages took seven years to pass, and it has been described as the most complex legislation in the Union's history and the most important in 20 years. It is the strictest law to date regulating chemical substances and will affect industries throughout the world. REACH entered into force on 1 June 2007, with a phased implementation over the next decade. The regulation also established the European Chemicals Agency, which manages the technical, scientific and administrative aspects of REACH. Overview When REACH is fully in force, it will require all companies manufacturing or importing chemical substances into the European Union in quantities of one tonne or more per year to register these substances with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dry Cleaning
Dry cleaning is any cleaning process for clothing and textiles using a solvent other than water. Dry cleaning still involves liquid, but clothes are instead soaked in a water-free liquid solvent. Tetrachloroethylene (perchloroethylene), known in the industry as "perc", is the most widely used solvent. Alternative solvents are 1-bromopropane and petroleum spirits. Most natural fibers can be washed in water but some synthetics (e.g., viscose, lyocell, modal, and cupro) react poorly with water and must be dry-cleaned. History Dry cleaning originated with American entrepreneur Thomas L. Jennings. Jennings referred to his method as “dry scouring”. French dye-works operator Jean Baptiste Jolly developed his own method using kerosene and gasoline to clean fabrics. He opened the first dry-cleaners in Paris in 1845. Flammability concerns led William Joseph Stoddard, a dry cleaner from Atlanta, to develop Stoddard solvent (white spirit) as a slightly less flammable alternati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perchloroethylene
Tetrachloroethylene, also known under the systematic name tetrachloroethene, or perchloroethylene, and many other names (and abbreviations such as "perc" or "PERC", and "PCE"), is a chlorocarbon with the formula Cl2C=CCl2 . It is a colorless liquid widely used for dry cleaning of fabrics, hence it is sometimes called "dry-cleaning fluid". It also has its uses as an effective automotive brake cleaner. It has a sweet odor detectable by most people at a concentration of 1 part per million (1 ppm). Worldwide production was about in 1985.M. Rossberg et al. "Chlorinated Hydrocarbons" in ''Ullmann's Encyclopedia of Industrial Chemistry'', 2006, Wiley-VCH, Weinheim. Production British physicist and chemist Michael Faraday first synthesized tetrachloroethylene in 1821 by thermal decomposition of hexachloroethane. :C2Cl6 → C2Cl4 + Cl2 Most tetrachloroethylene is produced by high temperature chlorinolysis of light hydrocarbons. The method is related to Faraday's discovery since hexach ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorofluorocarbon
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and propane. They are also commonly known by the DuPont brand name Freon. The most common representative is dichlorodifluoromethane (R-12 or Freon-12). Many CFCs have been widely used as refrigerants, propellants (in aerosol applications), and solvents. Because CFCs contribute to ozone depletion in the upper atmosphere, the manufacture of such compounds has been phased out under the Montreal Protocol, and they are being replaced with other products such as hydrofluorocarbons (HFCs) including R-410A and R-134a. Structure, properties and production As in simpler alkanes, carbon in the CFCs bond with tetrahedral symmetry. Because the fluorine and chlorine atoms differ greatly in size and effective charge from hydrogen and from each other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kauri-butanol Value
The Kauri-butanol value ("Kb value") is an international, standardized measure of solvent power for a hydrocarbon solvent, and is governed by an ASTM standardized test, ASTM D1133.ASTM D1133 - 10 Standard Test Method for Kauri-Butanol Value of Hydrocarbon Solvents The result of this test is a scaleless index, usually referred to as the "Kb value". A higher Kb value means the solvent is more aggressive or active in the ability to dissolve certain materials. Mild solvents have low scores in the tens and twenties; powerful solvents like chlorinated solvents and naphthenic aromatic solvents (i.e. "High Sol 10", "High Sol 15") have ratings that are in the low hundreds. In terms of the test itself, the kauri-butanol value (Kb) of a chemical shows the maximum amount of the hydrocarbon that can be added to a solution of kauri resin (a thick, gum-like material) in butanol (butyl alcohol) without causing cloudiness. Since kauri resin is readily soluble in butyl alcohol but not in most hydr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Degreasing
Degreasing, often called defatting or fat trimming, is the removal of fatty acids from an object. In culinary science, degreasing is done with the intention of reducing the fat content of a meal. Degreasing food Degreasing is often used by dieters, particularly those following low-fat diets to reduce their fat consumption to induce weight loss. The energy content of 1 g of fat is 9 calories, while that of carbohydrates and proteins are 4 calories. Hence, dieters often view decreasing fat consumption as an efficient way of losing weight without greatly sacrificing total volume of food. Degreasing during meal preparation is used to reduce the energy content of the food being prepared. Those people who wish to reduce their cholesterol level or fat intake, in particular people with hypercholesterolemia often use degreasing to reduce their fat consumption. Degreasing of a meal during preparation Fat trimming of a meal can be done during preparation by a variety of methods. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |