|
Michaelis–Menten Kinetics
In biochemistry, Michaelis–Menten kinetics is one of the best-known models of enzyme kinetics. It is named after German biochemist Leonor Michaelis and Canadian physician Maud Menten. The model takes the form of an equation describing the rate of enzymatic reactions, by relating reaction rate v (rate of formation of product, ce P/math>) to ce S/math>, the concentration of a substrate ''S''. Its formula is given by : v = \frac = V_\max \frac This equation is called the Michaelis–Menten equation. Here, V_\max represents the maximum rate achieved by the system, happening at saturating substrate concentration for a given enzyme concentration. When the value of the Michaelis constant K_\mathrm is numerically equal to the substrate concentration, then the reaction rate is half of V_\max. Biochemical reactions involving a single substrate are often assumed to follow Michaelis–Menten kinetics, without regard to the model's underlying assumptions. Model In 1901, French ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michaelis Menten Curve 2
Michaelis or Michelis is a surname. Notable people and characters with the surname include: * Adolf Michaelis, German classical scholar * Anthony R. Michaelis, German science writer * Edward Michelis, German theologian * Georg Michaelis, German politician * Gustav Adolf Michaelis, German obstetrician and namesake of the rhombus of Michaelis * Hans-Thorald Michaelis, German historian * Johann David Michaelis, German biblical scholar * John H. Michaelis, American four-star general * Laura Michaelis, American linguist * Leo Michelis, Greek-Canadian economist * Leonor Michaelis, German scientist known for Michaelis–Menten kinetics * Max Michaelis, South African financier * Margaret Michaelis-Sachs, Austrian-Australian photographer * Paul Charles Michaelis, American scientist * Peter Michaelis, German botanist * Robert Michaelis (1878–1965), French-born actor and singer who settled in England * Sebastian Michaelis, the demon butler from Kuroshitsuji * Sebastien Michaelis, French ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fructose
Fructose, or fruit sugar, is a Ketose, ketonic monosaccharide, simple sugar found in many plants, where it is often bonded to glucose to form the disaccharide sucrose. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed by the gut directly into the blood of the portal vein during digestion. The liver then converts both fructose and galactose into glucose, so that dissolved glucose, known as blood sugar, is the only monosaccharide present in circulating blood. Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847. The name "fructose" was coined in 1857 by the English chemist William Allen Miller. Pure, dry fructose is a sweet, white, odorless, crystalline solid, and is the most water-soluble of all the sugars. Fructose is found in honey, tree and vine fruits, flowers, Berry, berries, and most List of root vegetables, root vegetables. Commercially, fructose is derived from sugar cane, sugar beets, and maize. Hi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immune Complex
An immune complex, sometimes called an antigen-antibody complex or antigen-bound antibody, is a molecule formed from the binding of multiple antigens to antibodies. The bound antigen and antibody act as a unitary object, effectively an antigen of its own with a specific epitope. After an antigen-antibody reaction, the immune complexes can be subject to any of a number of responses, including complement deposition, opsonization, phagocytosis, or processing by proteases. Red blood cells carrying CR1-receptors on their surface may bind C3b-coated immune complexes and transport them to phagocytes, mostly in liver and spleen, and return to the general circulation. The ratio of antigen to antibody determines size and shape of immune complex. This, in turn, determines the effect of the immune complex. Many innate immune cells have FcRs, which are membrane-bound receptors that bind the constant regions of antibodies. Most FcRs on innate immune cells have low affinity for a singular ant ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Affinity (pharmacology)
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from ''ligare'', which means 'to bind'. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion, or protein which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure. Binding occurs by intermolecular forces, such as ionic bonds, hydrogen bonds and Van der Waals forces. The association or docking is actually reversible through dissociation. Measurably irreversible covalent bonding between a ligand and target molecule is atypical in biological systems. In contrast to the definition of liga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Turnover Number
Turnover number has two different meanings: In enzymology, turnover number (also termed ''k''cat) is defined as the maximum number of chemical conversions of substrate molecules per second that a single active site will execute for a given enzyme concentration _T/math> for enzymes with two or more active sites. For enzymes with a single active site, ''k''cat is referred to as the catalytic constant. It can be calculated from the maximum reaction rate V_\max and catalyst site concentration _T/math> as follows: :k_\mathrm = \frac (See Michaelis–Menten kinetics). In other chemical fields, such as organometallic catalysis, turnover number (abbreviated ''TON'') has a different meaning: the number of moles of substrate that a mole of catalyst can convert before becoming inactivated. An ideal catalyst would have an infinite turnover number in this sense, because it would never be consumed. The term turnover frequency (abbreviated ''TOF'') is used to refer to the turnover per unit tim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Asymptotically
In analytic geometry, an asymptote () of a curve is a line such that the distance between the curve and the line approaches zero as one or both of the ''x'' or ''y'' coordinates tends to infinity. In projective geometry and related contexts, an asymptote of a curve is a line which is tangent to the curve at a point at infinity. The word asymptote is derived from the Greek ἀσύμπτωτος (''asumptōtos'') which means "not falling together", from ἀ priv. + σύν "together" + πτωτ-ός "fallen". The term was introduced by Apollonius of Perga in his work on conic sections, but in contrast to its modern meaning, he used it to mean any line that does not intersect the given curve. There are three kinds of asymptotes: ''horizontal'', ''vertical'' and ''oblique''. For curves given by the graph of a function , horizontal asymptotes are horizontal lines that the graph of the function approaches as ''x'' tends to Vertical asymptotes are vertical lines near which the fu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Keith Laidler
Keith James Laidler (January 3, 1916 – August 26, 2003), born in England, was notable as a pioneer in chemical kinetics and authority on the physical chemistry of enzymes. Education Laidler received his early education at Liverpool College. He received his BA (1934) and MA (1938) degrees from Trinity College, Oxford University.Keith James Laidler, Physical Chemistry, A pioneer in the field of chemical kinetics and activated-complex theory The science.ca team, GCS Research Society (2001 and 2015).Keith J. Laidler (1916–2003) [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
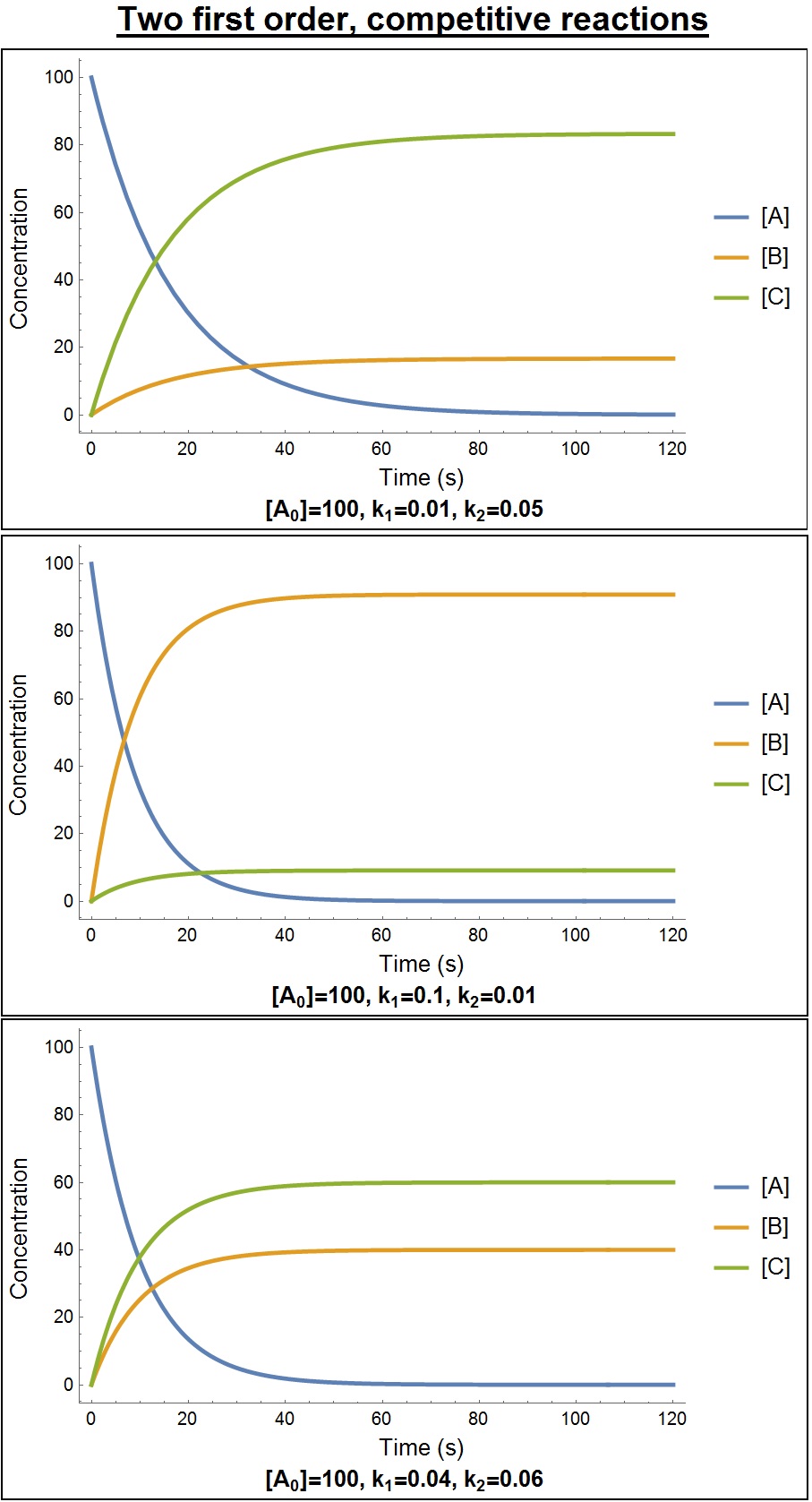
First-order Kinetics
In chemistry, the rate law or rate equation for a reaction is an equation that links the initial or forward reaction rate with the concentrations or pressures of the reactants and constant parameters (normally rate coefficients and partial reaction orders). For many reactions, the initial rate is given by a power law such as :v_0\; =\; k mathrmx mathrmy where and express the concentration of the species and usually in moles per liter (molarity, ). The exponents and are the partial ''orders of reaction'' for and and the ''overall'' reaction order is the sum of the exponents. These are often positive integers, but they may also be zero, fractional, or negative. The constant is the reaction rate constant or ''rate coefficient'' of the reaction. Its value may depend on conditions such as temperature, ionic strength, surface area of an adsorbent, or light irradiation. If the reaction goes to completion, the rate equation for the reaction rate v\; =\; k cex cey applies throughou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Order
In chemistry, the rate law or rate equation for a reaction is an equation that links the initial or forward reaction rate with the concentrations or pressures of the reactants and constant parameters (normally rate coefficients and partial reaction orders). For many reactions, the initial rate is given by a power law such as :v_0\; =\; k mathrmx mathrmy where and express the concentration of the species and usually in moles per liter (molarity, ). The exponents and are the partial ''orders of reaction'' for and and the ''overall'' reaction order is the sum of the exponents. These are often positive integers, but they may also be zero, fractional, or negative. The constant is the reaction rate constant or ''rate coefficient'' of the reaction. Its value may depend on conditions such as temperature, ionic strength, surface area of an adsorbent, or light irradiation. If the reaction goes to completion, the rate equation for the reaction rate v\; =\; k cex cey applies throu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Assumptions And Limitations
Assumption, in Christianity, refers to the Assumption of Mary, a belief in the taking up of the Virgin Mary into heaven. Assumption may also refer to: Places * Assumption, Alberta, Canada * Assumption, Illinois, United States ** Assumption Township, Christian County, Illinois * Assumption Island, Seychelles ** Assumption Island Airport * Assumption, Minnesota, United States * Assumption, Nebraska, United States * Assumption, Ohio, United States * Assumption Parish, Louisiana, United States Arts, entertainment, and media * "Assumption" (short story), a 1929 story by Samuel Beckett * Assumption of Moses, a Jewish apocryphal pseudepigraphical work of uncertain date and authorship Churches * Assumption Chapel, Minnesota, United States * Assumption of the Blessed Virgin Mary Church, Michigan, United States * Assumption – St. Paul, New York, United States * Cathedral of the Assumption (other) * Church of the Assumption (other) Logic * Closed-world assumption ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reversible Reaction
A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur simultaneously. : \mathit aA + \mathit bB \mathit cC + \mathit dD A and B can react to form C and D or, in the reverse reaction, C and D can react to form A and B. This is distinct from a reversible process in thermodynamics. Weak acids and bases undergo reversible reactions. For example, carbonic acid: : H2CO3 (l) + H2O(l) ⇌ HCO3−(aq) + H3O+(aq). The concentrations of reactants and products in an equilibrium mixture are determined by the analytical concentrations of the reagents (A and B or C and D) and the equilibrium constant, ''K''. The magnitude of the equilibrium constant depends on the Gibbs free energy change for the reaction. So, when the free energy change is large (more than about 30 kJ mol−1), the equilibrium constant is large (log K > 3) and the concentrations of the reactants at equilibrium are very small. Such a reac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Rate Constant
In chemical kinetics a reaction rate constant or reaction rate coefficient, ''k'', quantifies the rate and direction of a chemical reaction. For a reaction between reactants A and B to form product C the reaction rate is often found to have the form: r = k(T) mathrmm mathrm Here ''k''(''T'') is the reaction rate constant that depends on temperature, and and are the molar concentrations of substances A and B in moles per unit volume of solution, assuming the reaction is taking place throughout the volume of the solution. (For a reaction taking place at a boundary, one would use moles of A or B per unit area instead.) The exponents ''m'' and ''n'' are called partial orders of reaction and are ''not'' generally equal to the stoichiometric coefficients ''a'' and ''b''. Instead they depend on the reaction mechanism and can be determined experimentally. Elementary steps For an elementary step, there ''is'' a relationship between stoichiometry and rate law, as determined by th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |