|
Michael E. Jung
Michael E. Jung is a Professor of Chemistry in the Department of Chemistry and Biochemistry at the University of California at Los Angeles. Michael Jung was born May 14, 1947 in New Orleans, Louisiana. Early life and education Jung received a B.A. from Rice University in Houston, Texas in 1969 and a Ph.D. from Columbia University in New York City in 1973 where he did research with Gilbert Stork. Career Jung then obtained a NATO Postdoctoral Fellowship to work with Albert Eschenmoser at the Eidgenössische Technische Hochschule in Zürich, Switzerland. In 1974, he joined the faculty at UCLA, where he has spent his career. In 1979 Jung was awarded a Sloan research fellowship. Jung's research is focused on the development of new reactions for organic synthesis, including the Jung "non-aldol aldol" protocol, an alternate method for obtaining aldol products without using the classical aldol reaction. He has also developed chemical syntheses for a variety of natural products with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of California At Los Angeles
The University of California, Los Angeles (UCLA) is a public university, public Land-grant university, land-grant research university in Los Angeles, California. UCLA's academic roots were established in 1881 as a Normal school, teachers college then known as the southern branch of the California State Normal School (now San Jose State University, San José State University). This school was absorbed with the official founding of UCLA as the Southern Branch of the University of California in 1919, making it the second-oldest of the 10-campus University of California system (after UC Berkeley). UCLA offers 337 undergraduate and graduate degree programs in a wide range of disciplines, enrolling about 31,600 undergraduate and 14,300 graduate and professional students. UCLA received 174,914 undergraduate applications for Fall 2022, including transfers, making the school the most applied-to Higher education in the United States, university in the United States. The university is or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldol
In organic chemistry, an aldol describes a structural motif consisting of a 3-hydroxy ketone or 3-hydroxyaldehyde. Aldols are usually the product of aldol addition. When used alone, the term "aldol" may refer to 3-hydroxybutanal. Stereochemistry Despite having only two functional groups, many aldols can have complicated structures arising from the relative positions of the OH group and the substituent between it and the carbonyl. Controlling reactions in order to favor the formation of one of these isomers is of great interest in the area of asymmetric synthesis. Reactions The chemistry of aldols is dominated by one reaction, dehydration: :RC(O)CH2CH(OH)R' → RC(O)CH=CHR' + H2O Hydroxypivaldehyde Hydroxypivaldehyde is the organic compound with the formula HOCH2(CH3)2CCHO. A colorless liquid, it is produced by condensation of formaldehyde and isobutyraldehyde: :CH2O + (CH3)2CHCHO → HOCH2(CH3)2CCHO The compound is a rare example of a ... is a rare example of a rela ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
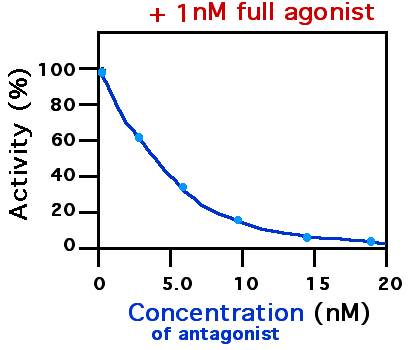
Receptor Antagonist
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins.Pharmacology Guide: In vitro pharmacology: concentration-response curves " '' GlaxoWellcome.'' Retrieved on December 6, 2007. They are sometimes called blockers; examples include alpha blockers, |
Apalutamide
Apalutamide, sold under the brand name Erleada among others, is a nonsteroidal antiandrogen (NSAA) medication which is used in the treatment of prostate cancer. It is specifically indicated for use in conjunction with castration in the treatment of non-metastatic castration-resistant prostate cancer (NM-CRPC). It is taken by mouth. Side effects of apalutamide when added to castration include fatigue, nausea, abdominal pain, diarrhea, high blood pressure, rash, falls, bone fractures, and an underactive thyroid. Rarely, it can cause seizures. The medication has a high potential for drug interactions. Apalutamide is an antiandrogen, and acts as an antagonist of the androgen receptor, the biological target of androgens like testosterone and dihydrotestosterone. In doing so, it prevents the effects of these hormones in the prostate gland and elsewhere in the body. Apalutamide was first described in 2007, and was approved for the treatment of prostate cancer in February 2018. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewis Acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. For example, NH3 is a Lewis base, because it can donate its lone pair of electrons. Trimethylborane (Me3B) is a Lewis acid as it is capable of accepting a lone pair. In a Lewis adduct, the Lewis acid and base share an electron pair furnished by the Lewis base, forming a dative bond. In the context of a specific chemical reaction between NH3 and Me3B, a lone pair from NH3 will form a dative bond with the empty orbital of Me3B to form an adduct NH3•BMe3. The terminology refers to the contributions of Gilbert N. Lewis. From p. 142: "We are inclined to think of substances as po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Robinson Annulation
The Robinson annulation is a chemical reaction used in organic chemistry for ring formation. It was discovered by Robert Robinson (organic chemist), Robert Robinson in 1935 as a method to create a six membered ring by forming three new carbon–carbon bonds. The method uses a ketone and a methyl vinyl ketone to form an α,β-unsaturated ketone in a cyclohexane ring by a Michael addition followed by an aldol condensation. This procedure is one of the key methods to form fused ring systems. Formation of cyclohexenone and derivatives are important in chemistry for their application to the synthesis of many natural products and other interesting organic compounds such as antibiotics and steroids. Specifically, the synthesis of cortisone is completed through the use of the Robinson annulation. The initial paper on the Robinson annulation was published by William Sage Rapson, William Rapson and Robert Robinson while Rapson studied at Oxford with professor Robinson. Before their work, c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halomon
Halomon is a polyhalogenated monoterpene first isolated from the marine red algae '' Portieria hornemannii''. Halomon has attracted research interest because of its promising profile of selective cytotoxicity that suggests its potential use as an antitumor agent. Halomon is in a class of chemical compounds known as halocarbons, which are often potent alkylating agents which may be toxic to individual cells or to living organisms. The red algae that naturally produce halomon and other related compounds probably do so as a poisonous defense against fish or other marine life that may see it as a potential source of food. Halomon, however, is a selective toxin; studies at the National Cancer Institute have indicated that it is more toxic to certain types of tumor cells than to other cells. The algae that produces halomon is difficult to locate, identify, and collect and the concentration of halomon in the organism is extremely low. Therefore, obtaining a sufficient amount of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antiviral Drug
Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Unlike most antibiotics, antiviral drugs do not destroy their target pathogen; instead they inhibit its development. Antiviral drugs are one class of antimicrobials, a larger group which also includes antibiotic (also termed antibacterial), antifungal and antiparasitic drugs, or antiviral drugs based on monoclonal antibodies. Most antivirals are considered relatively harmless to the host, and therefore can be used to treat infections. They should be distinguished from viricides, which are not medication but deactivate or destroy virus particles, either inside or outside the body. Natural viricides are produced by some plants such as eucalyptus and Australian tea trees. Medical uses Most of the antiviral drugs now available are designed to help deal with HIV, herpes viruses, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)